Timelines of translational science: From technology initiation to FDA approval
- PMID: 28481922
- PMCID: PMC5421779
- DOI: 10.1371/journal.pone.0177371
Timelines of translational science: From technology initiation to FDA approval
Abstract
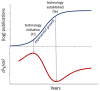
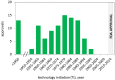
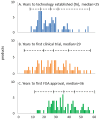
While timelines for clinical development have been extensively studied, there is little data on the broader path from initiation of research on novel drug targets, to approval of drugs based on this research. We examined timelines of translational science for 138 drugs and biologicals approved by the FDA from 2010-2014 using an analytical model of technology maturation. Research on targets for 102 products exhibited a characteristic (S-curve) maturation pattern with exponential growth between statistically defined technology initiation and established points. The median initiation was 1974, with a median of 25 years to the established point, 28 years to first clinical trials, and 36 years to FDA approval. No products were approved before the established point, and development timelines were significantly longer when the clinical trials began before this point (11.5 vs 8.5 years, p<0.0005). Technological maturation represents the longest stage of translation, and significantly impacts the efficiency of drug development.
Conflict of interest statement
Figures
References
-
- Woodcock J. The PCAST Report on Pharmaceutical Innovation: Implications for the FDA. Clinical Pharmacology & Therapeutics. 2013;94(3):297–300. - PubMed
-
- Sung NS, Crowley WF Jr, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. Jama. 2003;289(10):1278–87. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

