Evaluation of Nicotine Pharmacokinetics and Subjective Effects following Use of a Novel Nicotine Delivery System
- PMID: 28482017
- PMCID: PMC5896440
- DOI: 10.1093/ntr/ntx093
Evaluation of Nicotine Pharmacokinetics and Subjective Effects following Use of a Novel Nicotine Delivery System
Abstract
Introduction: Novel nicotine delivery systems represent an evolving part of the tobacco harm reduction strategy. The pharmacokinetic (PK) profile of nicotine delivered by P3L, a pulmonary nicotine delivery system, and its effects on smoking urges and craving relief in relation to Nicorette inhalator were evaluated.
Methods: This open-label, ascending nicotine levels study was conducted in 16 healthy smokers. Three different nicotine delivery levels, 50, 80, and 150 µg/puff, delivered by the P3L system were evaluated consecutively on different days after the use of the Nicorette inhalator. Venous nicotine PK, subjective effects, and tolerability were assessed.
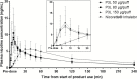
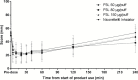
Results: Geometric least-squares means for maximum plasma nicotine concentration (Cmax), generated by the mixed-effect model for exposure comparison, were 9.7, 11.2, and 9.8 ng/mL for the 50, 80, and 150 µg/puff P3L variants, respectively, compared to 6.1 ng/mL after Nicorette inhalator use. Median time from product use start to Cmax was 7.0 minutes for all P3L, compared to 30.0 minutes for the Nicorette inhalator. Craving reduction was slightly faster than with the Nicorette inhalator as assessed with the visual analog scale craving score. The mean Questionnaire of Smoking Urges -brief total scores did not differ for both products. P3L was well tolerated.
Conclusions: At all three nicotine levels tested, the inhalation of the nicotine lactate aerosol delivered with the P3L provided plasma nicotine concentrations higher and faster compared to the Nicorette inhalator. The plasma nicotine concentration-time profile supports a pulmonary route of absorption for P3L compared to the oromucosal absorption of the Nicorette inhalator.
Implications: The combination of nicotine and lactic acid with the P3L device shows potential over existing nicotine delivery systems by delivering nicotine with kinetics close to published data on conventional cigarettes and without exogenous carrier substances as used in current electronic nicotine delivery systems. Altogether, the PK profile, subjective effects, and safety profile obtained in this study suggest P3L is an innovative nicotine delivery product that will be acceptable to adult smokers as an alternative to cigarettes.
Figures

References
-
- Royal College of Physicians. Harm Reduction in Nicotine Addiction: Helping People Who Can’t Quit. A report by the Tobacco Advisory Group of the Royal College of Physicians. London: RCP; 2007.
-
- Rose JE, Turner JE, Murugesan T, Behm FM, Laugesen M. Pulmonary delivery of nicotine pyruvate: sensory and pharmacokinetic characteristics. Exp Clin Psychopharmacol. 2010;18(5):385–394. doi:10.1037/a0020834. - PubMed
-
- Shahab L, Brose LS, West R. Novel delivery systems for nicotine replacement therapy as an aid to smoking cessation and for harm reduction: rationale, and evidence for advantages over existing systems. CNS Drugs. 2013;27(12):1007–1019. doi:10.1007/s40263-013-0116-4. - PubMed
-
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin. Pharmacol. Ther. 2008;83(4). doi:10.1038/clpt.2008.3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

