The effects of Alkanna tinctoria Tausch on split-thickness skin graft donor site management: a randomized, blinded placebo-controlled trial
- PMID: 28482839
- PMCID: PMC5422894
- DOI: 10.1186/s12906-017-1741-0
The effects of Alkanna tinctoria Tausch on split-thickness skin graft donor site management: a randomized, blinded placebo-controlled trial
Abstract
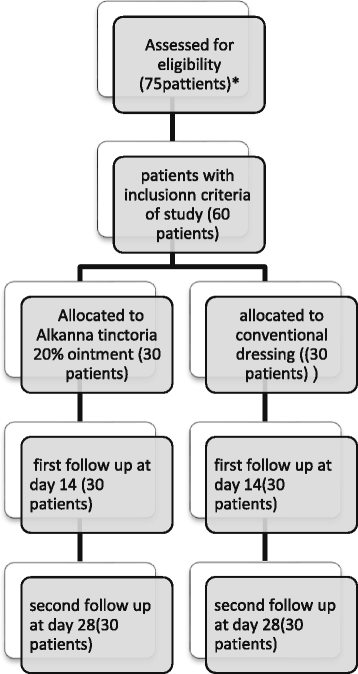
Background: A prospective, randomized, placebo-controlled clinical trial was conducted to compare the healing effectiveness of Alkanna tinctoria (L.) Tausch (Boraginaceae) with standard dressing on wound healing at the donor site after removal of the skin graft.
Methods: Enrolled patients were randomly allocated to receive topicalA. tinctoria extract ointment (20%) or standard dressing (dressing with base ointment) daily. Wound healing was assessed using the Bates-Jenson assessment tool at the 2nd and 4th weeks after intervention.
Results: Decreases in wound score were significantly greater in the A. tinctoria group compared with the placebo group (P <0.05). The surface areas of graft donor sites in the A. tinctoria group were significantly reduced as compared with the control group at day 28 of the intervention (P < 0.05). The proportion of patients in the A. tinctoria group achieving complete wound healing within 2 to 4 weeks was 50% and 96.66%, respectively, significantly higher than in patients receiving standard care: 0% and 23.3%, respectively.
Conclusions: This clinical study showed that A. tinctoria dressing accelerates wound healing after graft harvesting.
Trial registration: IRCT ID: IRCT201511165781N2 .
Keywords: Alkanna; Healing; Skin graft; Wound.
Figures
Similar articles
-
The effect of a beeswax, olive oil and Alkanna tinctoria (L.) Tausch mixture on burn injuries: An experimental study with a control group.Complement Ther Med. 2017 Oct;34:66-73. doi: 10.1016/j.ctim.2017.08.001. Epub 2017 Aug 10. Complement Ther Med. 2017. PMID: 28917377 Clinical Trial.
-
The effect of Alkanna tinctoria Tausch on burn wound healing in rabbits.Dtsch Tierarztl Wochenschr. 2002 Nov;109(11):481-5. Dtsch Tierarztl Wochenschr. 2002. PMID: 12494554
-
The Effects of Zataria multiflora Cream on Split-Thickness Skin Graft Donor-Site Management: A Randomized, Blinded, Placebo-Controlled Study.J Integr Complement Med. 2022 Dec;28(12):948-954. doi: 10.1089/jicm.2022.0533. Epub 2022 Oct 7. J Integr Complement Med. 2022. PMID: 36206040 Clinical Trial.
-
Accelerated reepithelialization by triterpenes: proof of concept in the healing of surgical skin lesions.Skin Pharmacol Physiol. 2015;28(1):1-11. doi: 10.1159/000357501. Epub 2014 Jul 15. Skin Pharmacol Physiol. 2015. PMID: 25034442 Clinical Trial.
-
Alkannins and shikonins: a new class of wound healing agents.Curr Med Chem. 2008;15(30):3248-67. doi: 10.2174/092986708786848532. Curr Med Chem. 2008. PMID: 19075667 Review.
Cited by
-
Co-Delivery of Dragon's Blood and Alkanna tinctoria Extracts Using Electrospun Nanofibers: In Vitro and In Vivo Wound Healing Evaluation in Diabetic Rat Model.Pharmaceutics. 2024 May 24;16(6):704. doi: 10.3390/pharmaceutics16060704. Pharmaceutics. 2024. PMID: 38931828 Free PMC article.
-
Reducing pain at split thickness donor sites with silicone dressing compared to petrolatum gauze dressing.Ann Burns Fire Disasters. 2019 Sep 30;32(3):210-215. Ann Burns Fire Disasters. 2019. PMID: 32313535 Free PMC article.
-
Trema orientale (L.) Blume: A review of its taxonomy, traditional uses, phytochemistry, pharmacological activities and domestication potential.Heliyon. 2023 Dec 13;10(1):e23640. doi: 10.1016/j.heliyon.2023.e23640. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38192795 Free PMC article. Review.
-
Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants.Mycorrhiza. 2022 Jul;32(3-4):221-256. doi: 10.1007/s00572-022-01079-0. Epub 2022 May 13. Mycorrhiza. 2022. PMID: 35556179 Free PMC article. Review.
-
Fabrication of Nanofibrous/Xerogel Layer-by-Layer Biocomposite Scaffolds for Skin Tissue Regeneration: In Vitro Study.ACS Omega. 2020 Jan 31;5(5):2133-2147. doi: 10.1021/acsomega.9b02832. eCollection 2020 Feb 11. ACS Omega. 2020. PMID: 32064374 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical