Critical attributes of human early mesenchymal stromal cell-laden microcarrier constructs for improved chondrogenic differentiation
- PMID: 28482913
- PMCID: PMC5421335
- DOI: 10.1186/s13287-017-0538-x
Critical attributes of human early mesenchymal stromal cell-laden microcarrier constructs for improved chondrogenic differentiation
Abstract
Background: Microcarrier cultures which are useful for producing large cell numbers can act as scaffolds to create stem cell-laden microcarrier constructs for cartilage tissue engineering. However, the critical attributes required to achieve efficient chondrogenic differentiation for such constructs are unknown. Therefore, this study aims to elucidate these parameters and determine whether cell attachment to microcarriers throughout differentiation improves chondrogenic outcomes across multiple microcarrier types.
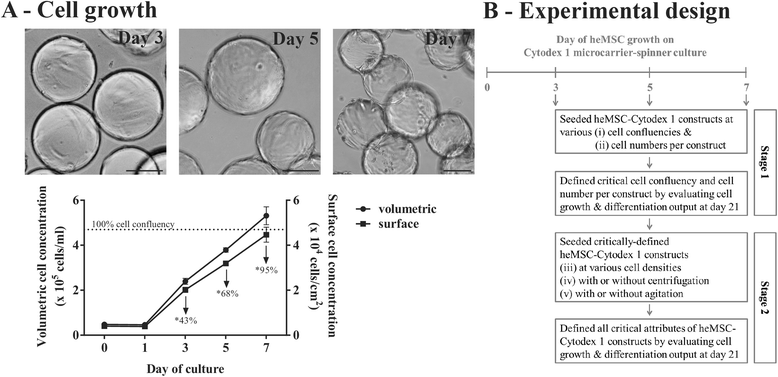
Methods: A screen was performed to evaluate whether 1) cell confluency, 2) cell numbers, 3) cell density, 4) centrifugation, or 5) agitation are crucial in driving effective chondrogenic differentiation of human early mesenchymal stromal cell (heMSC)-laden Cytodex 1 microcarrier (heMSC-Cytodex 1) constructs.
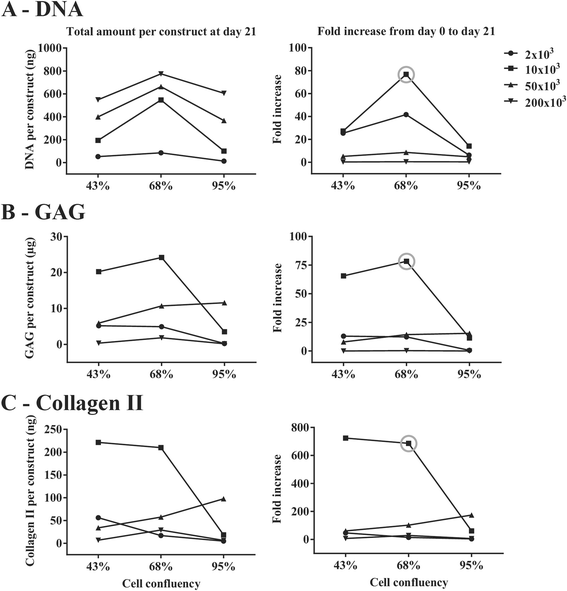
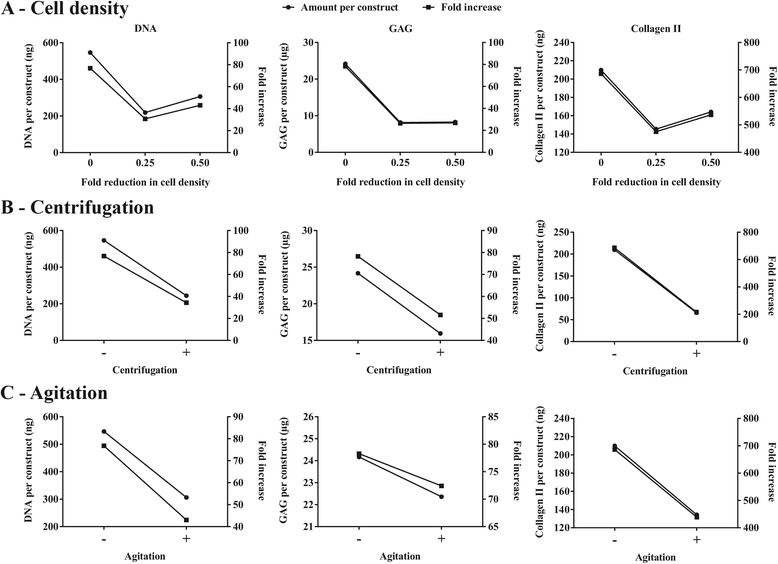
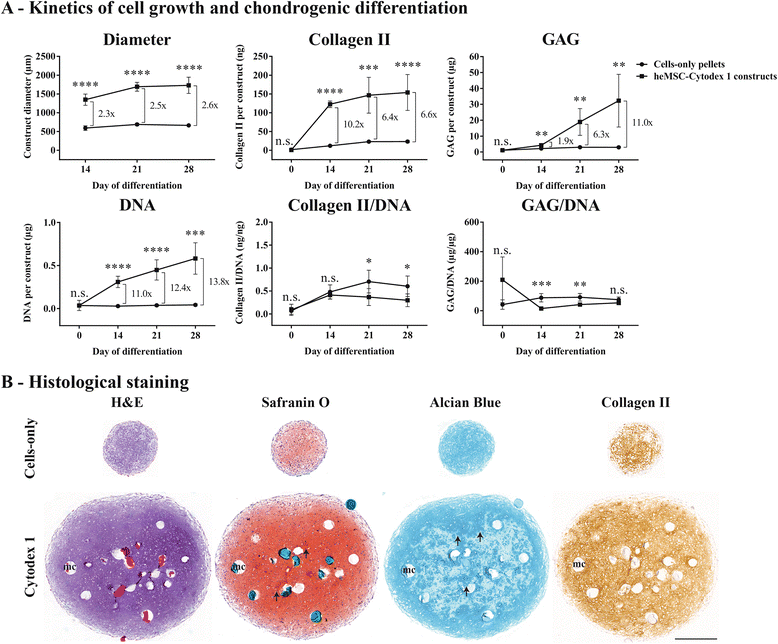
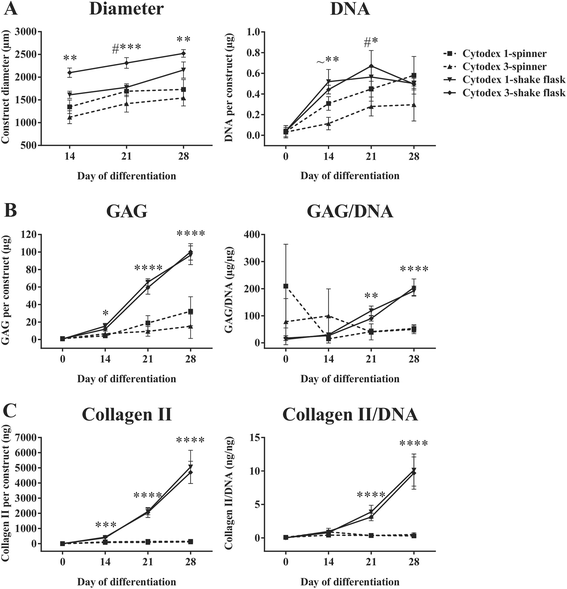
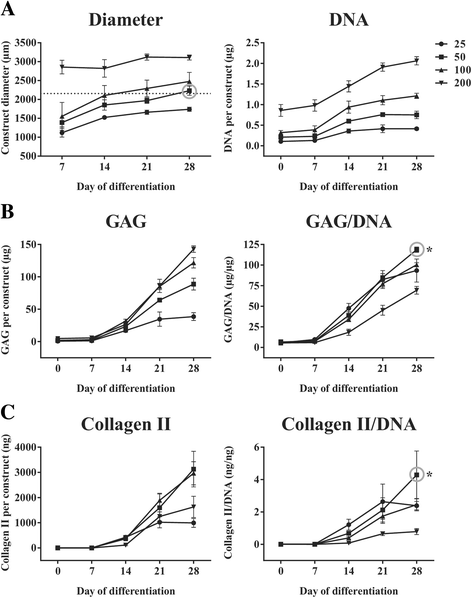
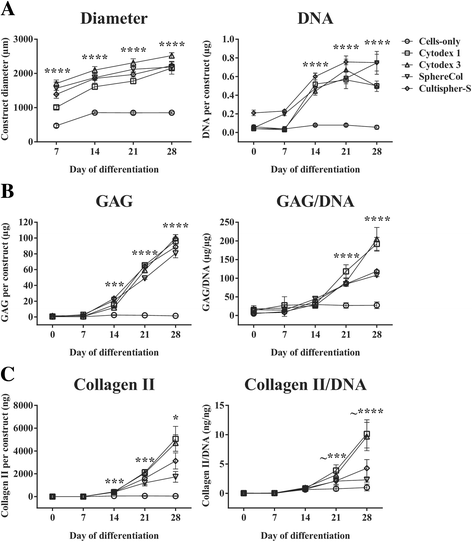
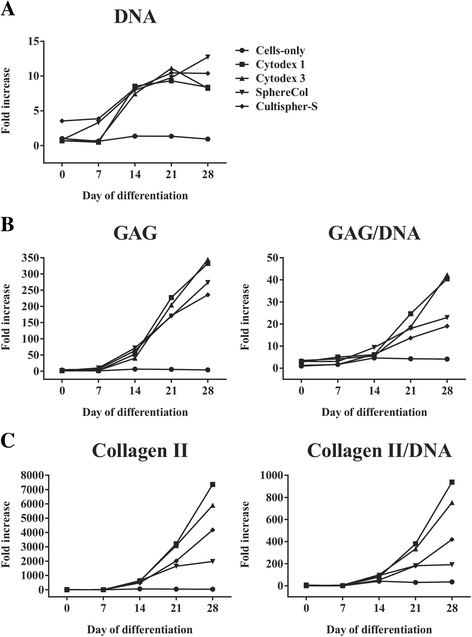
Results: Firstly, we found that seeding 10 × 103 cells at 70% cell confluency with 300 microcarriers per construct resulted in substantial increase in cell growth (76.8-fold increase in DNA) and chondrogenic protein generation (78.3- and 686-fold increase in GAG and Collagen II, respectively). Reducing cell density by adding empty microcarriers at seeding and indirectly compacting constructs by applying centrifugation at seeding or agitation throughout differentiation caused reduced cell growth and chondrogenic differentiation. Secondly, we showed that cell attachment to microcarriers throughout differentiation improves cell growth and chondrogenic outcomes since critically defined heMSC-Cytodex 1 constructs developed larger diameters (2.6-fold), and produced more DNA (13.8-fold), GAG (11.0-fold), and Collagen II (6.6-fold) than their equivalent cell-only counterparts. Thirdly, heMSC-Cytodex 1/3 constructs generated with cell-laden microcarriers from 1-day attachment in shake flask cultures were more efficient than those from 5-day expansion in spinner cultures in promoting cell growth and chondrogenic output per construct and per cell. Lastly, we demonstrate that these critically defined parameters can be applied across multiple microcarrier types, such as Cytodex 3, SphereCol and Cultispher-S, achieving similar trends in enhancing cell growth and chondrogenic differentiation.
Conclusions: This is the first study that has identified a set of critical attributes that enables efficient chondrogenic differentiation of heMSC-microcarrier constructs across multiple microcarrier types. It is also the first to demonstrate that cell attachment to microcarriers throughout differentiation improves cell growth and chondrogenic outcomes across different microcarrier types, including biodegradable gelatin-based microcarriers, making heMSC-microcarrier constructs applicable for use in allogeneic cartilage cell therapy.
Keywords: Cartilage; Cell therapy; Chondrogenic differentiation; Mesenchymal stromal cells; Microcarrier.
Figures
References
-
- Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–86. - PubMed
-
- Buckwalter JAMH. Articular cartilage, part 1: tissue design and chondrocyte-matrix interaction. J Bone Joint Surg Am. 1997;79:600–11. doi: 10.2106/00004623-199704000-00021. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

