Addressing Inpatient Beta-Lactam Allergies: A Multihospital Implementation
- PMID: 28483315
- PMCID: PMC5484001
- DOI: 10.1016/j.jaip.2017.02.019
Addressing Inpatient Beta-Lactam Allergies: A Multihospital Implementation
Abstract
Addressing inaccurate penicillin allergies is encouraged as part of antibiotic stewardship in the inpatient setting. However, implementing interventions targeted at the 10% to 15% of inpatients reporting a previous penicillin allergy can pose substantial logistic challenges. We implemented a computerized guideline for patients with reported beta-lactam allergy at 5 hospitals within a single health care system in the Boston area. In this article, we describe our implementation roadmap, including both successes achieved and challenges faced. We explain key implementation steps, including assembling a team, stakeholder engagement, developing or selecting an approach, spreading the change, establishing measures, and measuring impact. The objective was to detail the lessons learned while empowering others to be part of this important, multidisciplinary work to improve the care of patients with reported beta-lactam allergies.
Keywords: Adverse drug reaction; Allergy; Beta-lactam; Drug; Graded challenges; Guideline; Hypersensitivity; Penicillin; Policy; Quality improvement; Stewardship; Test dose.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


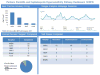
Type II–IV hypersensitivity reaction, avoidance of beta-lactams
Type I IgE-mediated hypersensitivity reaction or unknown reaction, 3rd/4th/5th generation cephalosporins can be used by test dose directly; to use 1st/2nd generation cephalosporins or penicillins, penicillin skin testing or desensitization and Allergy/Immunology follow up was recommended.
Mild hypersensitivity reaction including electronic health record discrepancies and benign morbilliform rashes, 3rd/4th/5th generation cephalosporins can be used by full dose and 1st/2nd generation cephalosporins and penicillins can be used by test dose.

Type II–IV hypersensitivity reaction, avoidance of beta-lactams
Type I IgE-mediated hypersensitivity reaction or unknown reaction, 3rd/4th/5th generation cephalosporins can be used by test dose directly; to use 1st/2nd generation cephalosporins or penicillins, penicillin skin testing or desensitization and Allergy/Immunology follow up was recommended.
Mild hypersensitivity reaction including electronic health record discrepancies and benign morbilliform rashes, 3rd/4th/5th generation cephalosporins can be used by full dose and 1st/2nd generation cephalosporins and penicillins can be used by test dose.

References
-
- Lee CE, Zembower TR, Fotis MA, Postelnick MJ, Greenberger PA, Peterson LR, et al. The incidence of antimicrobial allergies in hospitalized patients: implication regarding prescribing patters and emerging bacterial resistance. Arch Intern Med. 2000;160(18):2819–2822. - PubMed
-
- Picard M, Begin P, Bouchard H, Cloutier J, Lacombe-Barrios J, Paradis J, et al. Treatment of patients with a history of penicillin allergy in a large tertiary care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252–257. - PubMed
-
- Jeffres MN, Narayanan PP, Shuster JE, Schramm GE. Consequences of avoiding beta-lactams in patients with beta-lactam allergies. J Allergy Clin Immunol. 2016;137(4):1148–1153. - PubMed
-
- MacFadden DR, LaDelfa A, Leen J, Gold WL, Daneman N, Weber E, et al. Impact of Reported Beta-Lactam Allergy in Inpatient Outcomes: A Multicenter Prospective Cohort Study. Clin Infect Dis. 2016;63(7):904–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

