Therapeutic targeting of the HIF oxygen-sensing pathway: Lessons learned from clinical studies
- PMID: 28483447
- PMCID: PMC5507591
- DOI: 10.1016/j.yexcr.2017.05.004
Therapeutic targeting of the HIF oxygen-sensing pathway: Lessons learned from clinical studies
Abstract
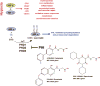
The oxygen-sensitive hypoxia-inducible factor (HIF) pathway plays a central role in the control of erythropoiesis and iron metabolism. The discovery of prolyl hydroxylase domain (PHD) proteins as key regulators of HIF activity has led to the development of inhibitory compounds that are now in phase 3 clinical development for the treatment of renal anemia, a condition that is commonly found in patients with advanced chronic kidney disease. This review provides a concise overview of clinical effects associated with pharmacologic PHD inhibition and was written in memory of Professor Lorenz Poellinger.
Keywords: Anemia; Chronic kidney disease; Clinical trials; Hypoxia; Hypoxia-inducible factor; Oxygen; Prolyl hydroxylase domain.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
VHH serves on the scientific advisory board of Akebia Therapeutics, Inc., a company that develops PHD inhibitors for the treatment of anemia
Figures
References
-
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78. - PubMed
-
- Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36:189–204. - PubMed
-
- Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. - PubMed
-
- Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. - PubMed
-
- Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

