Acetoacetate is a more efficient energy-yielding substrate for human mesenchymal stem cells than glucose and generates fewer reactive oxygen species
- PMID: 28483672
- PMCID: PMC5497396
- DOI: 10.1016/j.biocel.2017.05.007
Acetoacetate is a more efficient energy-yielding substrate for human mesenchymal stem cells than glucose and generates fewer reactive oxygen species
Abstract
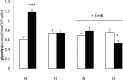
Stem cells have been assumed to demonstrate a reliance on anaerobic energy generation, suited to their hypoxic in vivo environment. However, we found that human mesenchymal stem cells (hMSCs) have an active oxidative metabolism with a range of substrates. More ATP was consistently produced from substrate oxidation than glycolysis by cultured hMSCs. Strong substrate preferences were shown with the ketone body, acetoacetate, being oxidised at up to 35 times the rate of glucose. ROS-generation was 45-fold lower during acetoacetate oxidation compared with glucose and substrate preference may be an adaptation to reduce oxidative stress. The UCP2 inhibitor, genipin, increased ROS production with either acetoacetate or glucose by 2-fold, indicating a role for UCP2 in suppressing ROS production. Addition of pyruvate stimulated acetoacetate oxidation and this combination increased ATP production 27-fold, compared with glucose alone, which has implications for growth medium composition. Oxygen tension during culture affected metabolism by hMSCs. Between passages 2 and 5, rates of both glycolysis and substrate-oxidation increased at least 2-fold for normoxic (20% O2)- but not hypoxic (5% O2)-cultured hMSCs, despite declining growth rates and no detectable signs of differentiation. Culture of the cells with 3-hydroxybutyrate abolished the increased rates of these pathways. These findings have implications for stem cell therapy, which necessarily involves in vitro culture of cells, since low passage number normoxic cultured stem cells show metabolic adaptations without detectable changes in stem-like status.
Keywords: Acetoacetate; Glycolysis; Oxidation; ROS; Stem cells.
Copyright © 2017. Published by Elsevier Ltd.
Figures






References
-
- Alt R., Riemer T., Fiehn O., Niederwieser D., Cross M. Evidence for restricted glycolytic metabolism in primary CD133+ cells. Blood. 2005;106 (Abs. 1726)
-
- Arsenijevic D., Onuma H., Percqueur C., Raimbault S., Manning B.S., Miroux B., Couplan E., Alves-Guerra M.C., Goubern M., Surwit R., Bouilaud F., Richard D., Collins S., Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435–439. - PubMed
-
- Bara J.J., Richards G.R., Alini M., Stoddart M. Concise review: bone marrow-Derived mesenchymal stem cells change phenotype following In vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32(7):1713–1723. - PubMed
-
- Bouillaud F. UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim. Biophys. Acta. 2009;1787:377–383. - PubMed
-
- Collins C.L., Bode B.P., Souba W.W., Abcouwer S.F. Multiwell 14CO2-capture assay for evaluation of substrate oxidation rates of cells in culture. Biotechniques. 1998;24(5):803–808. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

