Mobile App for Treatment of Stress Urinary Incontinence: A Cost-Effectiveness Analysis
- PMID: 28483745
- PMCID: PMC5440735
- DOI: 10.2196/jmir.7383
Mobile App for Treatment of Stress Urinary Incontinence: A Cost-Effectiveness Analysis
Abstract
Background: Mobile apps can increase access to care, facilitate self-management, and improve adherence to treatment. Stress urinary incontinence (SUI) affects 10-35% of women and, currently, an app with instructions for pelvic floor muscle training (PFMT) is available as first-line treatment. A previous randomized controlled study demonstrated that the app benefitted symptom severity and quality of life (QoL); in this study we investigate the cost-effectiveness of the app.
Objective: The objective of this study was to evaluate the health economy of the app for treating SUI.
Methods: This deterministic cost-utility analysis, with a 1-year societal perspective, compared the app treatment with no treatment. Health economic data were collected alongside a randomized controlled trial performed in Sweden from March 2013 to October 2014. This study included 123 community-dwelling women participants of 18 years and above, with stress urinary incontinence ≥1 time per week. Participants were self-assessed with validated questionnaires and 2-day leakage diaries, and then randomized to 3 months of treatment (app group, n=62) or no treatment (controls, n=61). The app focused on pelvic floor muscle training, prescribed 3 times daily. We continuously registered treatment delivery costs. Data were collected on each participant's training time, incontinence aids, and laundry at baseline and at a 3-month follow-up. We measured quality of life with the International Consultation on Incontinence Modular Questionnaire on Lower Urinary Tract Symptoms and Quality of Life, and calculated the quality-adjusted life years (QALYs) gained. Data from the 3-month follow-up were extrapolated to 1 year for the calculations. Our main outcome was the incremental cost-effectiveness ratios compared between app and control groups. One-way and multiway sensitivity analyses were performed.
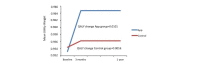
Results: The mean age of participants was 44.7 years (SD 9.4). Annual costs were €547.0 for the app group and €482.4 for the control group. Annual gains in quality-adjusted life years for app and control groups were 0.0101 and 0.0016, respectively. Compared with controls, the extra cost per quality-adjusted life year for the app group ranged from -€2425.7 to €14,870.6, which indicated greater gains in quality-adjusted life years at similar or slightly higher cost.
Conclusions: The app for treating stress urinary incontinence is a new, cost-effective, first-line treatment with potential for increasing access to care in a sustainable way for this patient group.
Keywords: cost-benefit analysis; mobile application; pelvic floor; self care; urinary incontinence, stress.
©Malin Sjöström, Lars Lindholm, Eva Samuelsson. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 08.05.2017.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
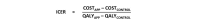
References
-
- WHO. From innovation to implementation http://www.euro.who.int/__data/assets/pdf_file/0012/302331/From-Innovati... .
-
- Whitehead L, Seaton P. The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-term Condition Management: A Systematic Review. J Med Internet Res. 2016;18(5):e97. doi: 10.2196/jmir.4883. http://www.jmir.org/2016/5/e97/ - DOI - PMC - PubMed
-
- Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52. doi: 10.2196/jmir.3951. http://www.jmir.org/2015/2/e52/ - DOI - PMC - PubMed
-
- Haylen BT, de RD, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN, International UA, International CS. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. doi: 10.1002/nau.20798. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

