Thyroid states regulate subcellular glucose phosphorylation activity in male mice
- PMID: 28483784
- PMCID: PMC5510448
- DOI: 10.1530/EC-17-0059
Thyroid states regulate subcellular glucose phosphorylation activity in male mice
Abstract
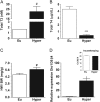
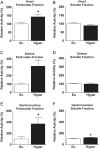
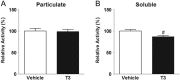
The thyroid hormones (THs), triiodothyronine (T3) and thyroxine (T4), are very important in organism metabolism and regulate glucose utilization. Hexokinase (HK) is responsible for the first step of glycolysis, catalyzing the conversion of glucose to glucose 6-phosphate. HK has been found in different cellular compartments, and new functions have been attributed to this enzyme. The effects of hyperthyroidism on subcellular glucose phosphorylation in mouse tissues were examined. Tissues were removed, subcellular fractions were isolated from eu- and hyperthyroid (T3, 0.25 µg/g, i.p. during 21 days) mice and HK activity was assayed. Glucose phosphorylation was increased in the particulate fraction in soleus (312.4% ± 67.1, n = 10), gastrocnemius (369.2% ± 112.4, n = 10) and heart (142.2% ± 13.6, n = 10) muscle in the hyperthyroid group compared to the control group. Hexokinase activity was not affected in brain or liver. No relevant changes were observed in HK activity in the soluble fraction for all tissues investigated. Acute T3 administration (single dose of T3, 1.25 µg/g, i.p.) did not modulate HK activity. Interestingly, HK mRNA levels remained unchanged and HK bound to mitochondria was increased by T3 treatment, suggesting a posttranscriptional mechanism. Analysis of the AKT pathway showed a 2.5-fold increase in AKT and GSK3B phosphorylation in the gastrocnemius muscle in the hyperthyroid group compared to the euthyroid group. Taken together, we show for the first time that THs modulate HK activity specifically in particulate fractions and that this action seems to be under the control of the AKT and GSK3B pathways.
Keywords: T3 action; glucose metabolism; mitochondria; muscle; thyroid.
© 2017 The authors.
Figures




Similar articles
-
Rapid changes in carbohydrate metabolism in muscle induced by triiodothyronine; the role of glucose 1,6-bisphosphate.Biochem Mol Med. 1995 Oct;56(1):19-25. doi: 10.1006/bmme.1995.1051. Biochem Mol Med. 1995. PMID: 8593533
-
The contribution of local thyroxine monodeiodination to intracellular 3,5, 3'-triiodothyronine in several tissues of hyperthyroid rats at isotopic equilibrium.Endocrinology. 1984 Jul;115(1):174-82. doi: 10.1210/endo-115-1-174. Endocrinology. 1984. PMID: 6734513
-
A multicompartmental model for iodide, thyroxine, and triiodothyronine metabolism in normal and spontaneously hyperthyroid cats.Endocrinology. 1988 Jun;122(6):2444-61. doi: 10.1210/endo-122-6-2444. Endocrinology. 1988. PMID: 3371254
-
Characterization of non-cytosolic hexokinase activity in white skeletal muscle from goldfish (Carassius auratus L.) and the effect of cold acclimation.Biosci Rep. 2010 Dec;30(6):413-23. doi: 10.1042/BSR20090128. Biosci Rep. 2010. PMID: 20055755
-
Mammalian hexokinases and their abnormal expression in cancer.Br J Biomed Sci. 2000;57(2):170-8. Br J Biomed Sci. 2000. PMID: 10912295 Review.
Cited by
-
Smoothelin-Like Protein 1 Regulates Development and Metabolic Transformation of Skeletal Muscle in Hyperthyroidism.Front Endocrinol (Lausanne). 2021 Oct 5;12:751488. doi: 10.3389/fendo.2021.751488. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34675885 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

