Imaging of viral neuroinvasion in the zebrafish reveals that Sindbis and chikungunya viruses favour different entry routes
- PMID: 28483796
- PMCID: PMC5536907
- DOI: 10.1242/dmm.029231
Imaging of viral neuroinvasion in the zebrafish reveals that Sindbis and chikungunya viruses favour different entry routes
Abstract
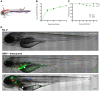
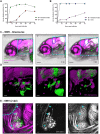
Alphaviruses, such as chikungunya virus (CHIKV) and Sindbis virus (SINV), are vector-borne pathogens that cause acute illnesses in humans and are sometimes associated with neuropathies, especially in infants and elderly patients. Little is known about their mechanism of entry into the central nervous system (CNS), even for SINV, which has been used extensively as a model for viral encephalopathies. We previously established a CHIKV infection model in the optically transparent zebrafish larva; here we describe a new SINV infection model in this host. We imaged in vivo the onset and progression of the infection caused by intravenous SINV inoculation. Similar to that described for CHIKV, infection in the periphery was detected early and was transient, whereas CNS infection started at later time points and was persistent or progressive. We then tested the possible mechanisms of neuroinvasion by CHIKV and SINV. Neither virus relied on macrophage-mediated transport to access the CNS. CHIKV, but not SINV, always infects endothelial cells of the brain vasculature. By contrast, axonal transport was much more efficient with SINV than CHIKV, both from the periphery to the CNS and between neural tissues. Thus, the preferred mechanisms of neuroinvasion by these two related viruses are distinct, providing a powerful imaging-friendly system to compare mechanisms and prevention methods of encephalopathies.
Keywords: Alphavirus; Central nervous system; Chikungunya; Live imaging; Viral encephalitis; Zebrafish.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




Similar articles
-
Novel Mutations in nsP2 Abolish Chikungunya Virus-Induced Transcriptional Shutoff and Make the Virus Less Cytopathic without Affecting Its Replication Rates.J Virol. 2019 Feb 5;93(4):e02062-18. doi: 10.1128/JVI.02062-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30487275 Free PMC article.
-
Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission.Antiviral Res. 2016 Nov;135:81-90. doi: 10.1016/j.antiviral.2016.10.003. Epub 2016 Oct 11. Antiviral Res. 2016. PMID: 27742486 Free PMC article.
-
Palmitoylated Cysteines in Chikungunya Virus nsP1 Are Critical for Targeting to Cholesterol-Rich Plasma Membrane Microdomains with Functional Consequences for Viral Genome Replication.J Virol. 2020 May 4;94(10):e02183-19. doi: 10.1128/JVI.02183-19. Print 2020 May 4. J Virol. 2020. PMID: 32132240 Free PMC article.
-
Cellular Attachment and Entry Factors for Chikungunya Virus.Viruses. 2019 Nov 19;11(11):1078. doi: 10.3390/v11111078. Viruses. 2019. PMID: 31752346 Free PMC article. Review.
-
Early Events in Chikungunya Virus Infection-From Virus Cell Binding to Membrane Fusion.Viruses. 2015 Jul 7;7(7):3647-74. doi: 10.3390/v7072792. Viruses. 2015. PMID: 26198242 Free PMC article. Review.
Cited by
-
Viral and Prion Infections Associated with Central Nervous System Syndromes in Brazil.Viruses. 2021 Jul 15;13(7):1370. doi: 10.3390/v13071370. Viruses. 2021. PMID: 34372576 Free PMC article. Review.
-
Effect of Viral Strain and Host Age on Clinical Disease and Viral Replication in Immunocompetent Mouse Models of Chikungunya Encephalomyelitis.Viruses. 2023 Apr 26;15(5):1057. doi: 10.3390/v15051057. Viruses. 2023. PMID: 37243143 Free PMC article.
-
Zebrafish: an underutilized tool for discovery in host-microbe interactions.Trends Immunol. 2022 Jun;43(6):426-437. doi: 10.1016/j.it.2022.03.011. Epub 2022 May 5. Trends Immunol. 2022. PMID: 35527182 Free PMC article. Review.
-
Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus.Cells. 2022 Apr 26;11(9):1457. doi: 10.3390/cells11091457. Cells. 2022. PMID: 35563763 Free PMC article.
-
Why Has the Ability to Regenerate Following CNS Injury Been Repeatedly Lost Over the Course of Evolution?Front Neurosci. 2022 Feb 4;16:831062. doi: 10.3389/fnins.2022.831062. eCollection 2022. Front Neurosci. 2022. PMID: 35185460 Free PMC article.
References
-
- Asakawa K., Suster M. L., Mizusawa K., Nagayoshi S., Kotani T., Urasaki A., Kishimoto Y., Hibi M. and Kawakami K. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 105, 1255-1260. 10.1073/pnas.0704963105 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

