Progerin-Induced Replication Stress Facilitates Premature Senescence in Hutchinson-Gilford Progeria Syndrome
- PMID: 28483909
- PMCID: PMC5492170
- DOI: 10.1128/MCB.00659-16
Progerin-Induced Replication Stress Facilitates Premature Senescence in Hutchinson-Gilford Progeria Syndrome
Abstract
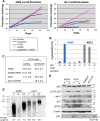
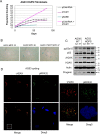
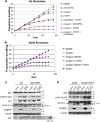
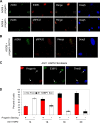
Hutchinson-Gilford progeria syndrome (HGPS) is caused by a mutation in LMNA that produces an aberrant lamin A protein, progerin. The accumulation of progerin in HGPS cells leads to an aberrant nuclear morphology, genetic instability, and p53-dependent premature senescence. How p53 is activated in response to progerin production is unknown. Here we show that young cycling HGPS fibroblasts exhibit chronic DNA damage, primarily in S phase, as well as delayed replication fork progression. We demonstrate that progerin binds to PCNA, altering its distribution away from replicating DNA in HGPS cells, leading to γH2AX formation, ATR activation, and RPA Ser33 phosphorylation. Unlike normal human cells that can be immortalized by enforced expression of telomerase alone, immortalization of HGPS cells requires telomerase expression and p53 repression. In addition, we show that the DNA damage response in HGPS cells does not originate from eroded telomeres. Together, these results establish that progerin interferes with the coordination of essential DNA replication factors, causing replication stress, and is the primary signal for p53 activation leading to premature senescence in HGPS. Furthermore, this damage response is shown to be independent of progerin farnesylation, implying that unprocessed lamin A alone causes replication stress.
Keywords: HGPS; aging; p53; progerin; senescence; telomere.
Copyright © 2017 American Society for Microbiology.
Figures




References
-
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. 2003. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423:293–298. doi: 10.1038/nature01629. - DOI - PMC - PubMed
-
- Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, Pei D, Pendas AM, Cadinanos J, Lopez-Otin C, Tse HF, Hutchison C, Chen J, Cao Y, Cheah KS, Tryggvason K, Zhou Z. 2005. Genomic instability in laminopathy-based premature aging. Nat Med 11:780–785. doi: 10.1038/nm1266. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
