Gepotidacin (GSK2140944) In Vitro Activity against Gram-Positive and Gram-Negative Bacteria
- PMID: 28483959
- PMCID: PMC5487655
- DOI: 10.1128/AAC.00468-17
Gepotidacin (GSK2140944) In Vitro Activity against Gram-Positive and Gram-Negative Bacteria
Abstract
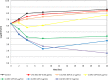
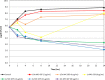
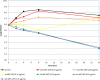
Gepotidacin is a first-in-class, novel triazaacenaphthylene antibiotic that inhibits bacterial DNA replication and has in vitro activity against susceptible and drug-resistant pathogens. Reference in vitro methods were used to investigate the MICs and minimum bactericidal concentrations (MBCs) of gepotidacin and comparator agents for Staphylococcus aureus, Streptococcus pneumoniae, and Escherichia coli Gepotidacin in vitro activity was also evaluated by using time-kill kinetics and broth microdilution checkerboard methods for synergy testing and for postantibiotic and subinhibitory effects. The MIC90 of gepotidacin for 50 S. aureus (including methicillin-resistant S. aureus [MRSA]) and 50 S. pneumoniae (including penicillin-nonsusceptible) isolates was 0.5 μg/ml, and for E. coli (n = 25 isolates), it was 4 μg/ml. Gepotidacin was bactericidal against S. aureus, S. pneumoniae, and E. coli, with MBC/MIC ratios of ≤4 against 98, 98, and 88% of the isolates tested, respectively. Time-kill curves indicated that the bactericidal activity of gepotidacin was observed at 4× or 10× MIC at 24 h for all of the isolates. S. aureus regrowth was observed in the presence of gepotidacin, and the resulting gepotidacin MICs were 2- to 128-fold higher than the baseline gepotidacin MICs. Checkerboard analysis of gepotidacin combined with other antimicrobials demonstrated no occurrences of antagonism with agents from multiple antimicrobial classes. The most common interaction when testing gepotidacin was indifference (fractional inhibitory concentration index of >0.5 to ≤4; 82.7% for Gram-positive isolates and 82.6% for Gram-negative isolates). The postantibiotic effect (PAE) of gepotidacin was short when it was tested against S. aureus (≤0.6 h against MRSA and MSSA), and the PAE-sub-MIC effect (SME) was extended (>8 h; three isolates at 0.5× MIC). The PAE of levofloxacin was modest (0.0 to 2.4 h), and the PAE-SME observed varied from 1.2 to >9 h at 0.5× MIC. These in vitro data indicate that gepotidacin is a bactericidal agent that exhibits a modest PAE and an extended PAE-SME against Gram-positive and -negative bacteria and merits further study for potential use in treating infections caused by these pathogens.
Keywords: gepotidacin; minimum bactericidal activity; postantibiotic effect.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, Huang J, Jones E, Jones J, Brown KK, Lewis CJ, May EW, Saunders MR, Singh O, Spitzfaden CE, Shen C, Shillings A, Theobald AJ, Wohlkonig A, Pearson ND, Gwynn MN. 2010. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466:935–940. doi:10.1038/nature09197. - DOI - PubMed
-
- Biedenbach DJ, Bouchillon SK, Hackel M, Miller LA, Scangarella-Oman NE, Jakielaszek C, Sahm DF. 2016. In vitro activity of gepotidacin, a novel triazaacenaphthylene bacterial topoisomerase inhibitor, against a broad spectrum of bacterial pathogens. Antimicrob Agents Chemother 60:1918–1923. doi:10.1128/AAC.02820-15. - DOI - PMC - PubMed
-
- Flamm RK, Sader HS, Rhomberg PR, Scangarella-Oman NE, Farrell DJ. 2016. Gepotidacin (GSK2140944) in vitro activity against Gram-positive and Gram-negative bacteria (MBC/MIC, kill kinetics, checkerboard, PAE/SME tests), abstr 460. ASM Microbe, Boston, MA, 16 to 20 June 2016.
-
- Farrell DJ, Sader HS, Rhomberg PR, Scangarella-Oman NE, Flamm RK. 2016. Gepotidacin (GSK2140944) in vitro activity against Neisseria gonorrhoeae (MIC/MBC, kill kinetics, checkerboard, PAE/SME tests), abstr 461. ASM Microbe, Boston, MA, 16 to 20 June 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases