Uncoupling of UNC5C with Polymerized TUBB3 in Microtubules Mediates Netrin-1 Repulsion
- PMID: 28483977
- PMCID: PMC5469302
- DOI: 10.1523/JNEUROSCI.2617-16.2017
Uncoupling of UNC5C with Polymerized TUBB3 in Microtubules Mediates Netrin-1 Repulsion
Abstract
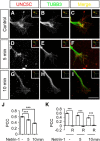
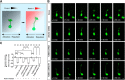
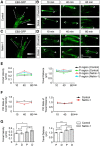
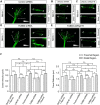
Modulation of microtubule (MT) dynamics is a key event of cytoskeleton remodeling in the growth cone (GC) during axon outgrowth and pathfinding. Our previous studies have shown that the direct interaction of netrin receptor DCC and DSCAM with polymerized TUBB3, a neuron-specific MT subunit in the brain, is required for netrin-1-mediated axon outgrowth, branching, and attraction. Here, we show that uncoupling of polymerized TUBB3 with netrin-1-repulsive receptor UNC5C is involved in netrin-1-mediated axonal repulsion. TUBB3 directly interacted with UNC5C and partially colocalized with UNC5C in the peripheral area of the GC of primary neurons from the cerebellar external granule layer of P2 mouse pups of both sexes. Netrin-1 reduced this interaction as well as the colocalization of UNC5C and TUBB3 in the GC. Results from the in vitro cosedimentation assay indicated that UNC5C interacted with polymerized TUBB3 in MTs and netrin-1 decreased this interaction. Knockdown of either TUBB3 or UNC5C blocked netrin-1-promoted axon repulsion in vitro and caused defects in axon projection of DRG toward the spinal cord in vivo Furthermore, live-cell imaging of end-binding protein 3 tagged with EGFP (EB3-GFP) in primary external granule layer cells showed that netrin-1 differentially increased MT dynamics in the GC with more MT growth in the distal than the proximal region of the GC during repulsion, and knockdown of either UNC5C or TUBB3 abolished the netrin-1 effect. Together, these data indicate that the disengagement of UNC5C with polymerized TUBB3 plays an essential role in netrin-1/UNC5C-mediated axon repulsion.SIGNIFICANCE STATEMENT Proper regulation of microtubule (MT) dynamics in the growth cone plays an important role in axon guidance. However, whether guidance cues modulate MT dynamics directly or indirectly is unclear. Here, we report that dissociation of UNC5C and polymerized TUBB3, the highly dynamic β-tubulin isoform in neurons, is essential for netrin-1/UNC5C-promoted axon repulsion. These results not only provide a working model of direct modulation of MTs by guidance cues in growth cone navigation but also help us to understand molecular mechanisms underlying developmental brain disorders associated with TUBB3 mutations.
Keywords: TUBB3; UNC5C; axon guidance; growth cone; microtubule dynamics; netrin.
Copyright © 2017 the authors 0270-6474/17/375620-14$15.00/0.
Figures



Similar articles
-
Coordinated interaction of Down syndrome cell adhesion molecule and deleted in colorectal cancer with dynamic TUBB3 mediates Netrin-1-induced axon branching.Neuroscience. 2015 May 7;293:109-22. doi: 10.1016/j.neuroscience.2015.02.042. Epub 2015 Mar 6. Neuroscience. 2015. PMID: 25754961 Free PMC article.
-
Direct binding of TUBB3 with DCC couples netrin-1 signaling to intracellular microtubule dynamics in axon outgrowth and guidance.J Cell Sci. 2013 Jul 15;126(Pt 14):3070-81. doi: 10.1242/jcs.122184. Epub 2013 May 2. J Cell Sci. 2013. PMID: 23641072 Free PMC article.
-
Disease-associated mutations in human TUBB3 disturb netrin repulsive signaling.PLoS One. 2019 Jun 21;14(6):e0218811. doi: 10.1371/journal.pone.0218811. eCollection 2019. PLoS One. 2019. PMID: 31226147 Free PMC article.
-
Role of Netrin-1 Signaling in Nerve Regeneration.Int J Mol Sci. 2017 Feb 24;18(3):491. doi: 10.3390/ijms18030491. Int J Mol Sci. 2017. PMID: 28245592 Free PMC article. Review.
-
Netrin, Slit and Wnt receptors allow axons to choose the axis of migration.Dev Biol. 2008 Nov 15;323(2):143-51. doi: 10.1016/j.ydbio.2008.08.027. Epub 2008 Sep 5. Dev Biol. 2008. PMID: 18801355 Review.
Cited by
-
Human TUBB3 Mutations Disrupt Netrin Attractive Signaling.Neuroscience. 2018 Mar 15;374:155-171. doi: 10.1016/j.neuroscience.2018.01.046. Epub 2018 Jan 31. Neuroscience. 2018. PMID: 29382549 Free PMC article.
-
Motor axon navigation relies on Fidgetin-like 1-driven microtubule plus end dynamics.J Cell Biol. 2018 May 7;217(5):1719-1738. doi: 10.1083/jcb.201604108. Epub 2018 Mar 13. J Cell Biol. 2018. PMID: 29535193 Free PMC article.
-
Investigation of dynamic solution interactions between NET-1 and UNC-5B by multi-wavelength analytical ultracentrifugation.Eur Biophys J. 2023 Jul;52(4-5):473-481. doi: 10.1007/s00249-023-01644-1. Epub 2023 Mar 20. Eur Biophys J. 2023. PMID: 36939874 Free PMC article.
-
Axonal Growth Abnormalities Underlying Ocular Cranial Nerve Disorders.Annu Rev Vis Sci. 2021 Sep 15;7:827-850. doi: 10.1146/annurev-vision-093019-114307. Epub 2021 Jun 3. Annu Rev Vis Sci. 2021. PMID: 34081534 Free PMC article. Review.
-
Arc Regulates Transcription of Genes for Plasticity, Excitability and Alzheimer's Disease.Biomedicines. 2022 Aug 11;10(8):1946. doi: 10.3390/biomedicines10081946. Biomedicines. 2022. PMID: 36009494 Free PMC article.
References
-
- Alcántara S, Ruiz M, De Castro F, Soriano E, Sotelo C (2000) Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development 127:1359–1372. - PubMed
-
- Bartoe JL, McKenna WL, Quan TK, Stafford BK, Moore JA, Xia J, Takamiya K, Huganir RL, Hinck L (2006) Protein interacting with C-kinase 1/protein kinase Calpha-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J Neurosci 26:3192–3205. 10.1523/JNEUROSCI.3469-05.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
