mTORC1 promotes proliferation of immature Schwann cells and myelin growth of differentiated Schwann cells
- PMID: 28484008
- PMCID: PMC5448230
- DOI: 10.1073/pnas.1620761114
mTORC1 promotes proliferation of immature Schwann cells and myelin growth of differentiated Schwann cells
Abstract
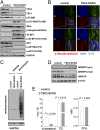
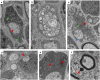
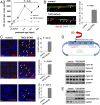
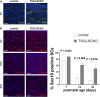
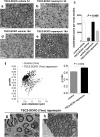
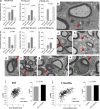
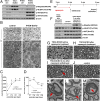
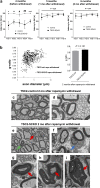
The myelination of axons in peripheral nerves requires precisely coordinated proliferation and differentiation of Schwann cells (SCs). We found that the activity of the mechanistic target of rapamycin complex 1 (mTORC1), a key signaling hub for the regulation of cellular growth and proliferation, is progressively extinguished as SCs differentiate during nerve development. To study the effects of different levels of sustained mTORC1 hyperactivity in the SC lineage, we disrupted negative regulators of mTORC1, including TSC2 or TSC1, in developing SCs of mutant mice. Surprisingly, the phenotypes ranged from arrested myelination in nerve development to focal hypermyelination in adulthood, depending on the level and timing of mTORC1 hyperactivity. For example, mice lacking TSC2 in developing SCs displayed hyperproliferation of undifferentiated SCs incompatible with normal myelination. However, these defects and myelination could be rescued by pharmacological mTORC1 inhibition. The subsequent reconstitution of SC mTORC1 hyperactivity in adult animals resulted in focal hypermyelination. Together our data suggest a model in which high mTORC1 activity promotes proliferation of immature SCs and antagonizes SC differentiation during nerve development. Down-regulation of mTORC1 activity is required for terminal SC differentiation and subsequent initiation of myelination. In distinction to this developmental role, excessive SC mTORC1 activity stimulates myelin growth, even overgrowth, in adulthood. Thus, our work delineates two distinct functions of mTORC1 in the SC lineage essential for proper nerve development and myelination. Moreover, our studies show that SCs retain their plasticity to myelinate and remodel myelin via mTORC1 throughout life.
Keywords: axon; mammalian target of rapamycin; myelination; neuropathy; peripheral nerve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Comment in
-
mTORC1 promotes Schwann cell cycling and myelinogenesis.Cell Cycle. 2017 Sep 17;16(18):1637-1638. doi: 10.1080/15384101.2017.1360635. Epub 2017 Aug 18. Cell Cycle. 2017. PMID: 28820327 Free PMC article. No abstract available.
Similar articles
-
Dual function of the PI3K-Akt-mTORC1 axis in myelination of the peripheral nervous system.Elife. 2017 Sep 7;6:e29241. doi: 10.7554/eLife.29241. Elife. 2017. PMID: 28880149 Free PMC article.
-
Exosomal miR-673-5p from fibroblasts promotes Schwann cell-mediated peripheral neuron myelination by targeting the TSC2/mTORC1/SREBP2 axis.J Biol Chem. 2022 Mar;298(3):101718. doi: 10.1016/j.jbc.2022.101718. Epub 2022 Feb 11. J Biol Chem. 2022. PMID: 35151688 Free PMC article.
-
Multiple lines of inhibitory feedback on AKT kinase in Schwann cells lacking TSC1/2 hint at distinct functions of mTORC1 and AKT in nerve development.Commun Integr Biol. 2018 Feb 12;11(1):e1433441. doi: 10.1080/19420889.2018.1433441. eCollection 2018. Commun Integr Biol. 2018. PMID: 29497474 Free PMC article.
-
The LKB1-AMPK and mTORC1 Metabolic Signaling Networks in Schwann Cells Control Axon Integrity and Myelination: Assembling and upholding nerves by metabolic signaling in Schwann cells.Bioessays. 2019 Jan;41(1):e1800075. doi: 10.1002/bies.201800075. Epub 2018 Dec 11. Bioessays. 2019. PMID: 30537168 Review.
-
Schwann Cell Development and Myelination.Cold Spring Harb Perspect Biol. 2024 Sep 3;16(9):a041360. doi: 10.1101/cshperspect.a041360. Cold Spring Harb Perspect Biol. 2024. PMID: 38503507 Review.
Cited by
-
Activation of mTORC1 signaling in gastric X/A-like cells induces spontaneous pancreatic fibrosis and derangement of glucose metabolism by reducing ghrelin production.EBioMedicine. 2018 Oct;36:304-315. doi: 10.1016/j.ebiom.2018.09.027. Epub 2018 Sep 25. EBioMedicine. 2018. PMID: 30266297 Free PMC article.
-
PIK3CA gain-of-function mutation in Schwann cells leads to severe neuropathy and aerobic glycolysis through a non-cell autonomous effect.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2424867122. doi: 10.1073/pnas.2424867122. Epub 2025 Jun 24. Proc Natl Acad Sci U S A. 2025. PMID: 40553493 Free PMC article.
-
Effects of NP-1 on proliferation, migration, and apoptosis of Schwann cell line RSC96 through the NF-κB signaling pathway.Am J Transl Res. 2020 Aug 15;12(8):4127-4140. eCollection 2020. Am J Transl Res. 2020. PMID: 32913493 Free PMC article.
-
Analysis of Signal Transduction Pathways Downstream M2 Receptor Activation: Effects on Schwann Cell Migration and Morphology.Life (Basel). 2022 Jan 29;12(2):211. doi: 10.3390/life12020211. Life (Basel). 2022. PMID: 35207498 Free PMC article.
-
Modulation of Schwann cell homeostasis by the BAP1 deubiquitinase.Glia. 2023 Jun;71(6):1466-1480. doi: 10.1002/glia.24351. Epub 2023 Feb 15. Glia. 2023. PMID: 36790040 Free PMC article.
References
-
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. - PubMed
-
- Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013;36:439–449. - PubMed
-
- Norrmén C, et al. mTORC1 controls PNS myelination along the mTORC1-RXRγ-SREBP-lipid biosynthesis axis in Schwann cells. Cell Reports. 2014;9:646–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

