Quorum Sensing Gene Regulation by LuxR/HapR Master Regulators in Vibrios
- PMID: 28484045
- PMCID: PMC5585708
- DOI: 10.1128/JB.00105-17
Quorum Sensing Gene Regulation by LuxR/HapR Master Regulators in Vibrios
Abstract
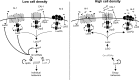
The coordination of group behaviors in bacteria is accomplished via the cell-cell signaling process called quorum sensing. Vibrios have historically been models for studying bacterial communication due to the diverse and remarkable behaviors controlled by quorum sensing in these bacteria, including bioluminescence, type III and type VI secretion, biofilm formation, and motility. Here, we discuss the Vibrio LuxR/HapR family of proteins, the master global transcription factors that direct downstream gene expression in response to changes in cell density. These proteins are structurally similar to TetR transcription factors but exhibit distinct biochemical and genetic features from TetR that determine their regulatory influence on the quorum sensing gene network. We review here the gene groups regulated by LuxR/HapR and quorum sensing and explore the targets that are common and unique among Vibrio species.
Keywords: HapR; LuxR; SmcR; Vibrio; Vibrio cholerae; Vibrio harveyi; gene regulation; quorum sensing.
Copyright © 2017 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

