Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes
- PMID: 28484079
- PMCID: PMC5461003
- DOI: 10.1084/jem.20161674
Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes
Abstract
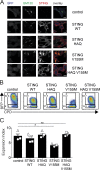
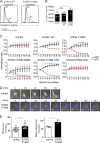
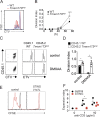
Activation of the cyclic dinucleotide sensor stimulator of interferon (IFN) genes (STING) is critical for IFN and inflammatory gene expression during innate immune responses. However, the role of STING in adaptive immunity is still unknown. In this study, we show that STING activation reduces the proliferation of T lymphocytes. This activity was independent of TBK1 and IRF3 recruitment and of type I IFN but required a distinct C-terminal domain of STING that activates NF-κB. Inhibition of cell proliferation by STING required its relocalization to the Golgi apparatus and caused mitotic errors. T lymphocytes from patients carrying constitutive active mutations in TMEM173 encoding STING showed impaired proliferation and reduced numbers of memory cells. Endogenous STING inhibited proliferation of mouse T lymphocytes. Therefore, STING, a critical innate sensor, also functions intrinsically in cells of the adaptive immune system to inhibit proliferation.
© 2017 Cerboni et al.
Figures





References
-
- Chien Y., Scuoppo C., Wang X., Fang X., Balgley B., Bolden J.E., Premsrirut P., Luo W., Chicas A., Lee C.S., et al. 2011. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 25:2125–2136. 10.1101/gad.17276711 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

