Characterizing microglia activation: a spatial statistics approach to maximize information extraction
- PMID: 28484229
- PMCID: PMC5431479
- DOI: 10.1038/s41598-017-01747-8
Characterizing microglia activation: a spatial statistics approach to maximize information extraction
Abstract
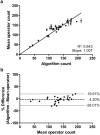
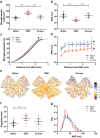
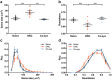
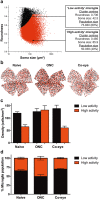
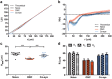
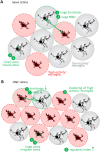
Microglia play an important role in the pathology of CNS disorders, however, there remains significant uncertainty about the neuroprotective/degenerative role of these cells due to a lack of techniques to adequately assess their complex behaviour in response to injury. Advancing microscopy techniques, transgenic lines and well-characterized molecular markers, have made histological assessment of microglia populations more accessible. However, there is a distinct lack of tools to adequately extract information from these images to fully characterise microglia behaviour. This, combined with growing economic pressures and the ethical need to minimise the use of laboratory animals, led us to develop tools to maximise the amount of information obtained. This study describes a novel approach, combining image analysis with spatial statistical techniques. In addition to monitoring morphological parameters and global changes in microglia density, nearest neighbour distance, and regularity index, we used cluster analyses based on changes in soma size and roundness to yield novel insights into the behaviour of different microglia phenotypes in a murine optic nerve injury model. These methods should be considered a generic tool to quantitatively assess microglia activation, to profile phenotypic changes into microglia subpopulations, and to map spatial distributions in virtually every CNS region and disease state.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

