Glial cell-line derived neurotrophic factor protects human islets from nutrient deprivation and endoplasmic reticulum stress induced apoptosis
- PMID: 28484241
- PMCID: PMC5431546
- DOI: 10.1038/s41598-017-01805-1
Glial cell-line derived neurotrophic factor protects human islets from nutrient deprivation and endoplasmic reticulum stress induced apoptosis
Abstract
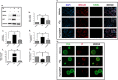
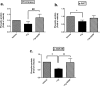
One of the key limitations to successful human islet transplantation is loss of islets due to stress responses pre- and post-transplantation. Nutrient deprivation and ER stress have been identified as important mechanisms leading to apoptosis. Glial Cell-line Derived Neurotrophic Factor (GDNF) has recently been found to promote islet survival after isolation. However, whether GDNF could rescue human islets from nutrient deprivation and ER stress-mediated apoptosis is unknown. Herein, by mimicking those conditions in vitro, we have shown that GDNF significantly improved glucose stimulated insulin secretion, reduced apoptosis and proinsulin:insulin ratio in nutrient deprived human islets. Furthermore, GDNF alleviated thapsigargin-induced ER stress evidenced by reduced expressions of IRE1α and BiP and consequently apoptosis. Importantly, this was associated with an increase in phosphorylation of PI3K/AKT and GSK3B signaling pathway. Transplantation of ER stressed human islets pre-treated with GDNF under kidney capsule of diabetic mice resulted in reduced expressions of IRE1α and BiP in human islet grafts with improved grafts function shown by higher levels of human C-peptide post-transplantation. We suggest that GDNF has protective and anti-apoptotic effects on nutrient deprived and ER stress activated human islets and could play a significant role in rescuing human islets from stress responses.
Conflict of interest statement
S.O.G is employed by AstraZeneca.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

