Tyrosine kinase inhibitors of Ripk2 attenuate bacterial cell wall-mediated lipolysis, inflammation and dysglycemia
- PMID: 28484277
- PMCID: PMC5431485
- DOI: 10.1038/s41598-017-01822-0
Tyrosine kinase inhibitors of Ripk2 attenuate bacterial cell wall-mediated lipolysis, inflammation and dysglycemia
Abstract
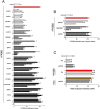
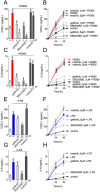
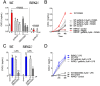
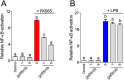
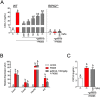
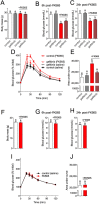
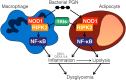
Inflammation underpins aspects of insulin resistance and dysglycemia. Microbiota-derived cell wall components such as muropeptides or endotoxin can trigger changes in host immunity and metabolism. Specific peptidoglycan motifs promote metabolic tissue inflammation, lipolysis and insulin resistance via Nucleotide-binding oligomerization domain-containing protein 1 (Nod1). Receptor-interacting serine/threonine-protein kinase 2 (Ripk2) mediates Nod1-induced immunity, but the role of Ripk2 in metabolism is ill-defined. We hypothesized that Ripk2 was required for Nod1-mediated inflammation, lipolysis and dysglycemia. This is relevant because certain tyrosine kinase inhibitors (TKIs) inhibit Ripk2 and there is clinical evidence of TKIs lowering inflammation and blood glucose. Here, we showed that only a subset of TKIs known to inhibit Ripk2 attenuated Nod1 ligand-mediated adipocyte lipolysis. TKIs that inhibit Ripk2 decreased cytokine responses induced by Nod1-activating peptidoglycan, but not endotoxin in both metabolic and immune cells. Pre-treatment of adipocytes or macrophages with the TKI gefitinib inhibited Nod1-induced Cxcl1 and Il-6 secretion. Furthermore, treatment of mice with gefitinib prevented Nod1-induced glucose intolerance in vivo. Ripk2 was required for these effects on inflammation and metabolism, since Nod1-mediated cytokine and blood glucose changes were absent in Ripk2-/- mice. Our data show that specific TKIs used in cancer also inhibit Nod1-Ripk2 immunometabolism responses indicative of metabolic disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
RIPK2 Dictates Insulin Responses to Tyrosine Kinase Inhibitors in Obese Male Mice.Endocrinology. 2020 Aug 1;161(8):bqaa086. doi: 10.1210/endocr/bqaa086. Endocrinology. 2020. PMID: 32473019
-
Bacterial peptidoglycan stimulates adipocyte lipolysis via NOD1.PLoS One. 2014 May 14;9(5):e97675. doi: 10.1371/journal.pone.0097675. eCollection 2014. PLoS One. 2014. PMID: 24828250 Free PMC article.
-
Postbiotics for NOD2 require nonhematopoietic RIPK2 to improve blood glucose and metabolic inflammation in mice.Am J Physiol Endocrinol Metab. 2020 Apr 1;318(4):E579-E585. doi: 10.1152/ajpendo.00033.2020. Epub 2020 Feb 26. Am J Physiol Endocrinol Metab. 2020. PMID: 32101030
-
RIPK2 NODs to XIAP and IBD.Semin Cell Dev Biol. 2021 Jan;109:144-150. doi: 10.1016/j.semcdb.2020.07.001. Epub 2020 Jul 3. Semin Cell Dev Biol. 2021. PMID: 32631784 Review.
-
Microbiota and Nod-like receptors balance inflammation and metabolism during obesity and diabetes.Biomed J. 2023 Oct;46(5):100610. doi: 10.1016/j.bj.2023.100610. Epub 2023 May 30. Biomed J. 2023. PMID: 37263539 Free PMC article. Review.
Cited by
-
NOD1-Targeted Immunonutrition Approaches: On the Way from Disease to Health.Biomedicines. 2021 May 6;9(5):519. doi: 10.3390/biomedicines9050519. Biomedicines. 2021. PMID: 34066406 Free PMC article. Review.
-
NOD-like Receptors-Emerging Links to Obesity and Associated Morbidities.Int J Mol Sci. 2023 May 11;24(10):8595. doi: 10.3390/ijms24108595. Int J Mol Sci. 2023. PMID: 37239938 Free PMC article. Review.
-
Activation of NOD1 and NOD2 in the development of liver injury and cancer.Front Immunol. 2022 Oct 4;13:1004439. doi: 10.3389/fimmu.2022.1004439. eCollection 2022. Front Immunol. 2022. PMID: 36268029 Free PMC article. Review.
-
NOD1: An Interface Between Innate Immunity and Insulin Resistance.Endocrinology. 2019 May 1;160(5):1021-1030. doi: 10.1210/en.2018-01061. Endocrinology. 2019. PMID: 30807635 Free PMC article. Review.
-
Inflammation promotes adipocyte lipolysis via IRE1 kinase.J Biol Chem. 2021 Jan-Jun;296:100440. doi: 10.1016/j.jbc.2021.100440. Epub 2021 Feb 19. J Biol Chem. 2021. PMID: 33610548 Free PMC article.
References
-
- Brooks MB. Erlotinib appears to produce prolonged remission of insulin-requiring type 2 diabetes associated with metabolic syndrome and chronic kidney disease. Br. J. Diabetes Vasc. Dis. 2012;12:87–90. doi: 10.1177/1474651412442694. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

