Multifunctional scanning ion conductance microscopy
- PMID: 28484332
- PMCID: PMC5415692
- DOI: 10.1098/rspa.2016.0889
Multifunctional scanning ion conductance microscopy
Abstract
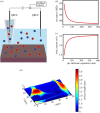
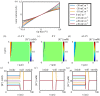
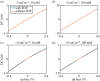
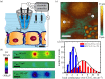
Scanning ion conductance microscopy (SICM) is a nanopipette-based technique that has traditionally been used to image topography or to deliver species to an interface, particularly in a biological setting. This article highlights the recent blossoming of SICM into a technique with a much greater diversity of applications and capability that can be used either standalone, with advanced control (potential-time) functions, or in tandem with other methods. SICM can be used to elucidate functional information about interfaces, such as surface charge density or electrochemical activity (ion fluxes). Using a multi-barrel probe format, SICM-related techniques can be employed to deposit nanoscale three-dimensional structures and further functionality is realized when SICM is combined with scanning electrochemical microscopy (SECM), with simultaneous measurements from a single probe opening up considerable prospects for multifunctional imaging. SICM studies are greatly enhanced by finite-element method modelling for quantitative treatment of issues such as resolution, surface charge and (tip) geometry effects. SICM is particularly applicable to the study of living systems, notably single cells, although applications extend to materials characterization and to new methods of printing and nanofabrication. A more thorough understanding of the electrochemical principles and properties of SICM provides a foundation for significant applications of SICM in electrochemistry and interfacial science.
Keywords: cellular imaging; charge mapping; electrochemical imaging; nanopipette; scanning ion conductance microscopy; single-cell analysis.
Conflict of interest statement
We declare we have no competing interests.
Figures










References
-
- Hansma PK, Drake B, Marti O, Gould SA, Prater CB. 1989. The scanning ion-conductance microscope. Science 243, 641–643. (doi:10.1126/science.2464851) - DOI - PubMed
-
- Korchev YE, Bashford CL, Milovanovic M, Vodyanoy I, Lab MJ. 1997. Scanning ion conductance microscopy of living cells. Biophys. J. 73, 653–658. (doi:10.1016/S0006-3495(97)78100-1) - DOI - PMC - PubMed
-
- Gorelik J, et al. 2004. The use of scanning ion conductance microscopy to image A6 cells. Mol. Cell. Endocrinol. 217, 101–108. (doi:10.1016/j.mce.2003.10.015) - DOI - PubMed
-
- Chen C-C, Zhou Y, Baker LA. 2012. Scanning ion conductance microscopy. Annu. Rev. Anal. Chem. 5, 207–228. (doi:10.1146/annurev-anchem-062011-143203) - DOI - PubMed
-
- Meyer E, Hug HJ, Bennewitz R. 2013. Scanning probe microscopy: the lab on a tip. Berlin, Germany: Springer Science & Business Media.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
