Aging Impairs Hippocampal- Dependent Recognition Memory and LTP and Prevents the Associated RyR Up-regulation
- PMID: 28484388
- PMCID: PMC5402473
- DOI: 10.3389/fnagi.2017.00111
Aging Impairs Hippocampal- Dependent Recognition Memory and LTP and Prevents the Associated RyR Up-regulation
Abstract
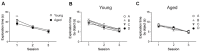
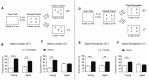
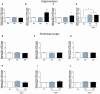
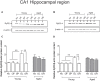
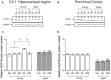
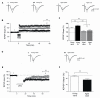
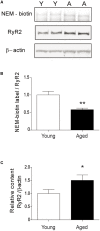
Recognition memory comprises recollection judgment and familiarity, two different processes that engage the hippocampus and the perirhinal cortex, respectively. Previous studies have shown that aged rodents display defective recognition memory and alterations in hippocampal synaptic plasticity. We report here that young rats efficiently performed at short-term (5 min) and long-term (24 h) hippocampus-associated object-location tasks and perirhinal cortex-related novel-object recognition tasks. In contrast, aged rats successfully performed the object-location and the novel-object recognition tasks only at short-term. In addition, aged rats displayed defective long-term potentiation (LTP) and enhanced long-term depression (LTD). Successful long-term performance of object-location but not of novel-object recognition tasks increased the protein levels of ryanodine receptor types-2/3 (RyR2/RyR3) and of IP3R1 in young rat hippocampus. Likewise, sustained LTP induction (1 h) significantly increased RyR2, RyR3 and IP3R1 protein levels in hippocampal slices from young rats. In contrast, LTD induction (1 h) did not modify the levels of these three proteins. Naïve (untrained) aged rats displayed higher RyR2/RyR3 hippocampal protein levels but similar IP3R1 protein content relative to young rats; these levels did not change following exposure to either memory recognition task or after LTP or LTD induction. The perirhinal cortex from young or aged rats did not display changes in the protein contents of RyR2, RyR3, and IP3R1 after exposure at long-term (24 h) to the object-location or the novel-object recognition tasks. Naïve aged rats displayed higher RyR2 channel oxidation levels in the hippocampus compared to naïve young rats. The RyR2/RyR3 up-regulation and the increased RyR2 oxidation levels exhibited by aged rat hippocampus are likely to generate anomalous calcium signals, which may contribute to the well-known impairments in hippocampal LTP and spatial memory that take place during aging.
Keywords: RyR oxidation; behavior; calcium release; gene expression; synaptic plasticity.
Figures



References
-
- Adasme T., Haeger P., Paula-Lima A. C., Espinoza I., Casas-Alarcon M. M., Carrasco M. A., et al. (2011). Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation. Proc. Natl. Acad. Sci. U.S.A. 108 3029–3034. 10.1073/pnas.1013580108 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

