Ocular Tremor in Parkinson's Disease: Discussion, Debate, and Controversy
- PMID: 28484420
- PMCID: PMC5401892
- DOI: 10.3389/fneur.2017.00134
Ocular Tremor in Parkinson's Disease: Discussion, Debate, and Controversy
Abstract
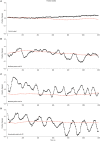
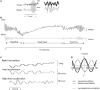
The identification of ocular tremor in a small cohort of patients with Parkinson's disease (PD) had lay somewhat dormant until the recent report of a pervasive ocular tremor as a universal finding in a large PD cohort that was, however, generally absent from a cohort of age-matched healthy subjects. The reported tremor had frequency characteristics similar to those of PD limb tremor, but the amplitude and frequency of the tremor did not correlate with clinical tremor ratings. Much controversy ensued as to the origin of such a tremor, and specifically as to whether a pervasive ocular tremor was a fundamental feature of PD, or rather a compensatory eye oscillation secondary to a transmitted head tremor, and thus a measure of a normal vestibulo-ocular reflex. In this mini review, we summarize some of the evidence for and against the case for a pervasive ocular tremor in PD and suggest future experiments that may help resolve these conflicting opinions.
Keywords: Parkinson’s disease; eye oscillations; head tremor; ocular tremor; vestibulo-ocular reflex.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

