Type I and Type III Interferons Display Different Dependency on Mitogen-Activated Protein Kinases to Mount an Antiviral State in the Human Gut
- PMID: 28484457
- PMCID: PMC5399069
- DOI: 10.3389/fimmu.2017.00459
Type I and Type III Interferons Display Different Dependency on Mitogen-Activated Protein Kinases to Mount an Antiviral State in the Human Gut
Abstract
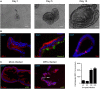
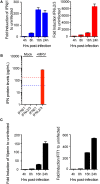
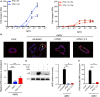
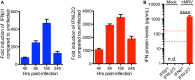
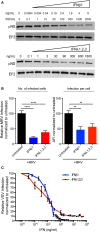
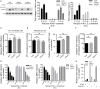
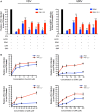
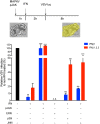
Intestinal epithelial cells (IECs) are constantly exposed to commensal flora and pathogen challenges. How IECs regulate their innate immune response to maintain gut homeostasis remains unclear. Interferons (IFNs) are cytokines produced during infections. While type I IFN receptors are ubiquitously expressed, type III IFN receptors are expressed only on epithelial cells. This epithelium specificity strongly suggests exclusive functions at epithelial surfaces, but the relative roles of type I and III IFNs in the establishment of an antiviral innate immune response in human IECs are not clearly defined. Here, we used mini-gut organoids to define the functions of types I and III IFNs to protect the human gut against viral infection. We show that primary non-transformed human IECs, upon viral challenge, upregulate the expression of both type I and type III IFNs at the transcriptional level but only secrete type III IFN in the supernatant. However, human IECs respond to both type I and type III IFNs by producing IFN-stimulated genes that in turn induce an antiviral state. Using genetic ablation of either type I or type III IFN receptors, we show that either IFN can independently restrict virus infection in human IECs. Importantly, we report, for the first time, differences in the mechanisms by which each IFN establishes the antiviral state. Contrary to type I IFN, the antiviral activity induced by type III IFN is strongly dependent on the mitogen-activated protein kinases signaling pathway, suggesting a pathway used by type III IFNs that non-redundantly contributes to the antiviral state. In conclusion, we demonstrate that human intestinal epithelial cells specifically regulate their innate immune response favoring type III IFN-mediated signaling, which allows for efficient protection against pathogens without producing excessive inflammation. Our results strongly suggest that type III IFN constitutes the frontline of antiviral response in the human gut. We propose that mucosal surfaces, particularly the gastrointestinal tract, have evolved to favor type III IFN-mediated response to pathogen infections as it allows for spatial segregation of signaling and moderate production of inflammatory signals which we propose are key to maintain gut homeostasis.
Keywords: antiviral immunity; human gut microbiota; interferon-lambda; interferon-β; intestinal epithelial cells; mitogen-activated protein kinases; mucosal immunity.
Figures
Similar articles
-
Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus.J Virol. 2017 Mar 13;91(7):e02079-16. doi: 10.1128/JVI.02079-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28077655 Free PMC article.
-
Type III and Not Type I Interferons Efficiently Prevent the Spread of Rotavirus in Human Intestinal Epithelial Cells.J Virol. 2022 Sep 14;96(17):e0070622. doi: 10.1128/jvi.00706-22. Epub 2022 Aug 24. J Virol. 2022. PMID: 36000839 Free PMC article.
-
Selective Interferon Responses of Intestinal Epithelial Cells Minimize Tumor Necrosis Factor Alpha Cytotoxicity.J Virol. 2020 Oct 14;94(21):e00603-20. doi: 10.1128/JVI.00603-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32847859 Free PMC article.
-
Importance of Type I and III Interferons at Respiratory and Intestinal Barrier Surfaces.Front Immunol. 2020 Dec 11;11:608645. doi: 10.3389/fimmu.2020.608645. eCollection 2020. Front Immunol. 2020. PMID: 33362795 Free PMC article. Review.
-
Herpesviruses and the Type III Interferon System.Virol Sin. 2021 Aug;36(4):577-587. doi: 10.1007/s12250-020-00330-2. Epub 2021 Jan 5. Virol Sin. 2021. PMID: 33400088 Free PMC article. Review.
Cited by
-
Astrocyte Control of Zika Infection Is Independent of Interferon Type I and Type III Expression.Biology (Basel). 2022 Jan 15;11(1):143. doi: 10.3390/biology11010143. Biology (Basel). 2022. PMID: 35053142 Free PMC article.
-
Advances in understanding interferon-mediated immune responses to enteric viruses in intestinal organoids.Front Immunol. 2022 Jul 22;13:943334. doi: 10.3389/fimmu.2022.943334. eCollection 2022. Front Immunol. 2022. PMID: 35935957 Free PMC article. Review.
-
Using Diverse Model Systems to Define Intestinal Epithelial Defenses to Enteric Viral Infections.Cell Host Microbe. 2020 Mar 11;27(3):329-344. doi: 10.1016/j.chom.2020.02.003. Cell Host Microbe. 2020. PMID: 32164844 Free PMC article. Review.
-
Hypoxic Environment Promotes Barrier Formation in Human Intestinal Epithelial Cells through Regulation of MicroRNA 320a Expression.Mol Cell Biol. 2019 Jun 27;39(14):e00553-18. doi: 10.1128/MCB.00553-18. Print 2019 Jul 15. Mol Cell Biol. 2019. PMID: 31061092 Free PMC article.
-
The Role of Interferon in Persistent Viral Infection: Insights from Murine Norovirus.Trends Microbiol. 2018 Jun;26(6):510-524. doi: 10.1016/j.tim.2017.10.010. Epub 2017 Nov 17. Trends Microbiol. 2018. PMID: 29157967 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

