The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases
- PMID: 28484466
- PMCID: PMC5402045
- DOI: 10.3389/fimmu.2017.00475
The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases
Abstract
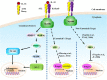
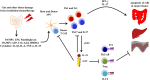
Il1rl1 (also known as ST2) is a member of the IL-1 superfamily, and its only known ligand is IL-33. ST2 exists in two forms as splice variants: a soluble form (sST2), which acts as a decoy receptor, sequesters free IL-33, and does not signal, and a membrane-bound form (ST2), which activates the MyD88/NF-κB signaling pathway to enhance mast cell, Th2, regulatory T cell (Treg), and innate lymphoid cell type 2 functions. sST2 levels are increased in patients with active inflammatory bowel disease, acute cardiac and small bowel transplant allograft rejection, colon and gastric cancers, gut mucosal damage during viral infection, pulmonary disease, heart disease, and graft-versus-host disease. Recently, sST2 has been shown to be secreted by intestinal pro-inflammatory T cells during gut inflammation; on the contrary, protective ST2-expressing Tregs are decreased, implicating that ST2/IL-33 signaling may play an important role in intestinal disease. This review will focus on what is known on its signaling during various inflammatory disease states and highlight potential avenues to intervene in ST2/IL-33 signaling as treatment options.
Keywords: IL-33; IL1RL1; ST2; cardiac diseases; graft-versus-host disease; lung diseases.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

