Mitochondrial DNA Hypomethylation Is a Biomarker Associated with Induced Senescence in Human Fetal Heart Mesenchymal Stem Cells
- PMID: 28484495
- PMCID: PMC5397648
- DOI: 10.1155/2017/1764549
Mitochondrial DNA Hypomethylation Is a Biomarker Associated with Induced Senescence in Human Fetal Heart Mesenchymal Stem Cells
Abstract
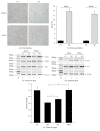
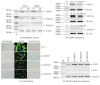
Background. Fetal heart can regenerate to restore its normal anatomy and function in response to injury, but this regenerative capacity is lost within the first week of postnatal life. Although the specific molecular mechanisms remain to be defined, it is presumed that aging of cardiac stem or progenitor cells may contribute to the loss of regenerative potential. Methods. To study this aging-related dysfunction, we cultured mesenchymal stem cells (MSCs) from human fetal heart tissues. Senescence was induced by exposing cells to chronic oxidative stress/low serum. Mitochondrial DNA methylation was examined during the period of senescence. Results. Senescent MSCs exhibited flattened and enlarged morphology and were positive for the senescence-associated beta-galactosidase (SA-β-Gal). By scanning the entire mitochondrial genome, we found that four CpG islands were hypomethylated in close association with senescence in MSCs. The mitochondrial COX1 gene, which encodes the main subunit of the cytochrome c oxidase complex and contains the differentially methylated CpG island 4, was upregulated in MSCs in parallel with the onset of senescence. Knockdown of DNA methyltransferases (DNMT1, DNMT3a, and DNMT3B) also upregulated COX1 expression and induced cellular senescence in MSCs. Conclusions. This study demonstrates that mitochondrial CpG hypomethylation may serve as a critical biomarker associated with cellular senescence induced by chronic oxidative stress.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis.Stem Cell Res Ther. 2018 Sep 26;9(1):257. doi: 10.1186/s13287-018-0970-6. Stem Cell Res Ther. 2018. PMID: 30257725 Free PMC article.
-
FGF21 Mediates Mesenchymal Stem Cell Senescence via Regulation of Mitochondrial Dynamics.Oxid Med Cell Longev. 2019 Apr 17;2019:4915149. doi: 10.1155/2019/4915149. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31178962 Free PMC article.
-
Alteration of fatty acid oxidation by increased CPT1A on replicative senescence of placenta-derived mesenchymal stem cells.Stem Cell Res Ther. 2020 Jan 3;11(1):1. doi: 10.1186/s13287-019-1471-y. Stem Cell Res Ther. 2020. PMID: 31900237 Free PMC article.
-
Quantifying Senescence-Associated Phenotypes in Primary Multipotent Mesenchymal Stromal Cell Cultures.Methods Mol Biol. 2019;2045:93-105. doi: 10.1007/7651_2019_217. Methods Mol Biol. 2019. PMID: 31020633
-
Aging of the cells: Insight into cellular senescence and detection Methods.Eur J Cell Biol. 2020 Aug;99(6):151108. doi: 10.1016/j.ejcb.2020.151108. Epub 2020 Jul 12. Eur J Cell Biol. 2020. PMID: 32800277 Review.
Cited by
-
Senescent mesenchymal stem/stromal cells and restoring their cellular functions.World J Stem Cells. 2020 Sep 26;12(9):966-985. doi: 10.4252/wjsc.v12.i9.966. World J Stem Cells. 2020. PMID: 33033558 Free PMC article. Review.
-
Associations between Circulating Biomarkers of One-Carbon Metabolism and Mitochondrial D-Loop Region Methylation Levels.Epigenomes. 2024 Oct 9;8(4):38. doi: 10.3390/epigenomes8040038. Epigenomes. 2024. PMID: 39449362 Free PMC article.
-
Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages.Neural Regen Res. 2018 Dec;13(12):2182-2190. doi: 10.4103/1673-5374.241469. Neural Regen Res. 2018. PMID: 30323151 Free PMC article.
-
Aging of mesenchymal stem cell: machinery, markers, and strategies of fighting.Cell Mol Biol Lett. 2022 Aug 19;27(1):69. doi: 10.1186/s11658-022-00366-0. Cell Mol Biol Lett. 2022. PMID: 35986247 Free PMC article. Review.
-
The Prospective Application of Melatonin in Treating Epigenetic Dysfunctional Diseases.Front Pharmacol. 2022 May 20;13:867500. doi: 10.3389/fphar.2022.867500. eCollection 2022. Front Pharmacol. 2022. PMID: 35668933 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

