How do red blood cells know when to die?
- PMID: 28484605
- PMCID: PMC5414242
- DOI: 10.1098/rsos.160850
How do red blood cells know when to die?
Abstract
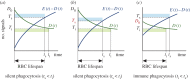
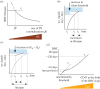
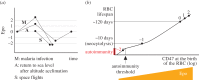
Human red blood cells (RBCs) are normally phagocytized by macrophages of splenic and hepatic sinusoids at 120 days of age. The destruction of RBCs is ultimately controlled by antagonist effects of phosphatidylserine (PS) and CD47 on the phagocytic activity of macrophages. In this work, we introduce a conceptual model that explains RBC lifespan as a consequence of the dynamics of these molecules. Specifically, we suggest that PS and CD47 define a molecular algorithm that sets the timing of RBC phagocytosis. We show that significant changes in RBC lifespan described in the literature can be explained as alternative outcomes of this algorithm when it is executed in different conditions of oxygen availability. The theoretical model introduced here provides a unified framework to understand a variety of empirical observations regarding RBC biology. It also highlights the role of RBC lifespan as a key element of RBC homeostasis.
Keywords: CD47; erythropoietin; neocytolysis; oxygen homeostasis; phosphatidylserine; red blood cell homeostasis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Wynn TA, Chawla A, Pollard JW. 2013. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455. (doi:10.1038/nature12034) - DOI - PMC - PubMed
-
- Lang E, Qadri SM, Lang F. 2012. Killing me softly—suicidal erythrocyte death. Int. J. Biochem. Cell Biol. 44, 1236–1243. (doi:10.1016/j.biocel.2012.04.019) - DOI - PubMed
-
- Trial J, Rice L. 2004. Erythropoietin withdrawal leads to the destruction of young red cells at the endothelial-macrophage interface. Curr. Pharmaceut. Des. 10, 183–190. (doi:10.2174/1381612043453423) - DOI - PubMed
-
- Goodman JW, Smith LH. 1961. Erythrocyte life span in normal mice and in radiation bone marrow chimeras. Am. J. Physiol. 200, 764–770. - PubMed
-
- Horký J, Vácha J, Znojil V. 1977. Comparison of life span of erythrocytes in some inbred strains of mouse using 14C-labelled glycine. Physiol. Bohemoslovaca 27, 209–217. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

