Subcutaneous and Sublingual Immunotherapy in Allergic Asthma in Children
- PMID: 28484690
- PMCID: PMC5399038
- DOI: 10.3389/fped.2017.00082
Subcutaneous and Sublingual Immunotherapy in Allergic Asthma in Children
Erratum in
-
Corrigendum: Subcutaneous and Sublingual Immunotherapy in Allergic Asthma in Children.Front Pediatr. 2017 Sep 11;5:187. doi: 10.3389/fped.2017.00187. eCollection 2017. Front Pediatr. 2017. PMID: 28936430 Free PMC article.
Abstract
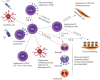
This review presents up-to-date understanding of immunotherapy in the treatment of children with allergic asthma. The principal types of allergen immunotherapy (AIT) are subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). Both of them are indicated for patients with allergic rhinitis and/or asthma, who have evidence of clinically relevant allergen-specific IgE, and significant symptoms despite reasonable avoidance measures and/or maximal medical therapy. Studies have shown a significant decrease in asthma symptom scores and in the use of rescue medication, and a preventive effect on asthma onset. Although the safety profile of SLIT appears to be better than SCIT, the results of some studies and meta-analyses suggest that the efficacy of SCIT is better and that SCIT has an earlier onset than SLIT in children with allergic asthma. Severe, not controlled asthma, and medical error were the most frequent causes of SCIT-induced adverse events.
Keywords: allergy; asthma; children; subcutaneous immunotherapy; sublingual immunotherapy.
Figures
References
-
- Burks AWC, Casale MA, Cox T, Demoly P, Jutel M, Nelson H, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol (2013) 131(5):1288–96.e3. 10.1016/j.jaci.2013.01.049 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

