Urinary transcript quantitation of CK20 and IGF2 for the non-invasive bladder cancer detection
- PMID: 28484844
- PMCID: PMC11818997
- DOI: 10.1007/s00432-017-2433-3
Urinary transcript quantitation of CK20 and IGF2 for the non-invasive bladder cancer detection
Abstract
Purpose: Cytokeratin 20 (CK20) and insulin-like growth factor 2 (IGF2) were previously proposed to be elevated in clinical samples from patients with bladder cancer (BCa). A two cohort design validation study was used to assess the relevance for BCa detection by transcript quantitation of both markers in urine samples. Their diagnostic value was assessed in comparison with voided urine cytology (VUC).
Methods: RNA isolation was carried out using cellular sediments of urine samples from 196/103 histologically positive BCa patients, as well as 97/50 control subjects for the test (TC) and validation cohort (VC), respectively. Urinary transcript levels of CK20 and IGF2 were determined by qPCR.
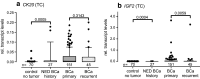
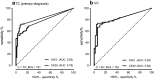
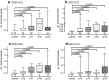
Results: Relative transcript levels were significantly elevated 3.4/11-fold for CK20 and 188/64-fold for IGF2 (p < 0.001) in urine sediments of BCa patients compared to controls in the TC and VC, respectively. In a combined analysis, the resulting sensitivity (SN) (SNTC: 77.9; SNVC: 90.3%) and specificity (SP) (SPTC: 88.0; SPVC: 84.0%) were similar to that of VUC. The sensitivity of VUC in combination with CK20 and IGF2 was considerably increased (SNTC: 94.6; SNVC: 93.2%) while specificity was reduced (SPTC: 72.0; SPVC: 82.0%) compared to VUC alone in the test and validation cohort.
Conclusions: Transcript levels of IGF2 and CK20 enabled the detection of BCa with a diagnostic performance similar to VUC. Combined analysis of voided urine cytology together with altered transcript levels of CK20 and IGF2 enhanced sensitivity, but did not improve overall test performance.
Keywords: Cytokeratin 20; Insulin-like growth factor II; KRT20; Tumor marker; Urine; Urothelial carcinoma.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

