A randomized phase II study of standard-dose versus high-dose rituximab with BEAM in autologous stem cell transplantation for relapsed aggressive B-cell non-hodgkin lymphomas: long term results
- PMID: 28485023
- PMCID: PMC5672904
- DOI: 10.1111/bjh.14731
A randomized phase II study of standard-dose versus high-dose rituximab with BEAM in autologous stem cell transplantation for relapsed aggressive B-cell non-hodgkin lymphomas: long term results
Abstract
High-dose rituximab (HD-R) combined with carmustine, cytarabine, etoposide and melphalan (BEAM) and autologous stem cell transplant (ASCT) was effective and tolerable in a single-arm prospective study of relapsed aggressive B-cell non-Hodgkin lymphoma (R-NHL). We performed a randomized phase 2 study comparing HD-R versus standard-dose rituximab (SD-R) in R-NHL. Ninety-three patients were randomized to HD-R (1000 mg/m2 ) (n = 42) or SD-R (375 mg/m2 ) (n = 51) administered on post-transplant days +1 and +8, using a Bayesian adaptive algorithm. The 2 treatment arms were balanced in regards to patient demographic and clinical characteristics. At a median follow-up of 7·92 years, the 5-year disease-free survival (DFS) and overall survival (OS) were 40% and 48%, respectively. We found no statistically significant differences between HD-R and SD-R in 5-year DFS (36% vs. 43%; P = 0·205) and OS (43% vs. 52%; P = 0·392). In multivariate analyses, only disease status before ASCT [residual disease versus complete remission (CR)] (hazard ratio [HR] 1·79, 95% confidence interval [CI]: 1·08-2·95) and number of prior treatments received (>2 vs. ≤2 lines of treatment) (HR 1·89, 95% CI: 1·13-3·18) were associated with worse DFS and OS. Patients who had SCT while in CR or who received ≤2 lines of treatment prior to SCT had better 5-year OS (57% vs. 35%; P = 0·02 and 54% vs. 30%, P = 0·001, respectively) in both arms. No differences in engraftments or adverse events were noted in the 2 arms. When combined with BEAM and ASCT in relapsed aggressive B-cell NHL, HD-R provided no DFS or OS advantage over SD-R. In patients who have been exposed to rituximab in the frontline or salvage setting, the addition of rituximab in the peri-transplant setting remains controversial.
Keywords: autologous transplant; carmustine, cytarabine, etoposide and melphalan; non-Hodgkin lymphoma; rituximab.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
Figures
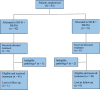

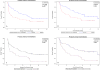
References
-
- Berry DA, Eick SG. Adaptive assignment versus balanced randomization in clinical trials: a decision analysis. Stat Med. 1995;14:231–246. - PubMed
-
- Brugger W, Hirsch J, Grunebach F, Repp R, Brossart P, Vogel W, Kopp HG, Manz MG, Bitzer M, Schlimok G, Kaufmann M, Ganser A, Fehnle K, Gramatzki M, Kanz L. Rituximab consolidation after high-dose chemotherapy and autologous blood stem cell transplantation in follicular and mantle cell lymphoma: a prospective, multicenter phase II study. Ann Oncol. 2004;15:1691–1698. - PubMed
-
- Chen YB, Lane AA, Logan BR, Zhu X, Akpek G, Aljurf MD, Artz AS, Bredeson CN, Cooke KR, Ho VT, Lazarus HM, Olsson RF, Saber W, McCarthy PL, Pasquini MC. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:1046–1053. - PMC - PubMed
-
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. - PubMed
-
- Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, Rubinger M, Buckstein R, Imrie KR, Federico M, Di Renzo N, Howson-Jan K, Baetz T, Kaizer L, Voralia M, Olney HJ, Turner AR, Sussman J, Hay AE, Djurfeldt MS, Meyer RM, Chen BE, Shepherd LE. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32:3490–3496. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

