Improved cognitive outcomes in patients with relapsing-remitting multiple sclerosis treated with daclizumab beta: Results from the DECIDE study
- PMID: 28485186
- PMCID: PMC5971365
- DOI: 10.1177/1352458517707345
Improved cognitive outcomes in patients with relapsing-remitting multiple sclerosis treated with daclizumab beta: Results from the DECIDE study
Abstract
Background: Cognitive impairment is common in multiple sclerosis (MS), with cognitive processing speed being the most frequently affected domain.
Objective: Examine the effects of daclizumab beta versus intramuscular (IM) interferon (IFN) beta-1a on cognitive processing speed as assessed by Symbol Digit Modalities Test (SDMT).
Methods: In DECIDE, patients with relapsing-remitting multiple sclerosis (RRMS) (age: 18-55 years; Expanded Disability Status Scale (EDSS) score 0-5.0) were randomized to daclizumab beta ( n = 919) or IM IFN beta-1a ( n = 922) for 96-144 weeks. SDMT was administered at baseline and at 24-week intervals.
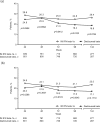
Results: At week 96, significantly greater mean improvement from baseline in SDMT was observed with daclizumab beta versus IM IFN beta-1a ( p = 0.0274). Significantly more patients treated with daclizumab beta showed clinically meaningful improvement in SDMT (increase from baseline of ⩾3 points ( p = 0.0153) or ⩾4 points ( p = 0.0366)), and significantly fewer patients showed clinically meaningful worsening (decrease from baseline of ⩾3 points ( p = 0.0103)). Odds representing risk of worsening versus stability or improvement on SDMT were significantly smaller for daclizumab beta ( p = 0.0088 (3-point threshold); p = 0.0267 (4-point threshold)). In patients completing 144 weeks of treatment, the effects of daclizumab beta were generally sustained.
Conclusion: These results provide evidence for a benefit of daclizumab beta versus IM IFN beta-1a on cognitive processing speed in RRMS.
Trial registration: ClinicalTrials.gov identifier NCT01064401 (Efficacy and Safety of BIIB019 (Daclizumab High Yield Process) Versus Interferon β 1a in Participants With Relapsing-Remitting Multiple Sclerosis (DECIDE)): https://clinicaltrials.gov/ct2/show/NCT01064401 .
Keywords: Clinical trial; cognitive impairments; daclizumab; interferon beta-1a; multiple sclerosis; phase III; randomized controlled trial.
Conflict of interest statement
Figures



References
-
- Costa SL, Genova HM, DeLuca J, et al. Information processing speed in multiple sclerosis: Past, present, and future. Mult Scler 2017; 23: 772–789. - PubMed
-
- Rao SM, Leo GJ, Haughton VM, et al. Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology 1989; 39: 161–166. - PubMed
-
- Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 1991; 41: 692–696. - PubMed
-
- Fischer JS, Priore RL, Jacobs LD, et al. Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol 2000; 48: 885–892. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

