Adenosine A2A receptor inactivation alleviates early-onset cognitive dysfunction after traumatic brain injury involving an inhibition of tau hyperphosphorylation
- PMID: 28485728
- PMCID: PMC5534966
- DOI: 10.1038/tp.2017.98
Adenosine A2A receptor inactivation alleviates early-onset cognitive dysfunction after traumatic brain injury involving an inhibition of tau hyperphosphorylation
Abstract
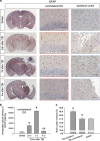
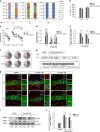
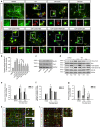
Tau is a microtubule-associated protein, and the oligomeric and hyperphosphorylated forms of tau are increased significantly after neurotrauma and considered important factors in mediating cognitive dysfunction. Blockade of adenosine A2A receptors, either by caffeine or gene knockout (KO), alleviates cognitive dysfunction after traumatic brain injury (TBI). We postulated that A2AR activation exacerbates cognitive impairment via promoting tau hyperphosphorylation. Using a mouse model of moderate controlled cortical impact, we showed that TBI induced hyperphosphorylated tau (p-tau) in the hippocampal dentate gyrus and spatial memory deficiency in the Morris water maze test at 7 days and 4 weeks after TBI. Importantly, pharmacological blockade (A2AR antagonist ZM241385 or non-selective adenosine receptor antagonist caffeine) or genetic inactivation of A2ARs reduced the level of tau phosphorylation at Ser404 and alleviated spatial memory dysfunction. The A2AR control of p-tau is further supported by the observations that a KO of A2AR decreased the activity of the tau phosphorylation kinases, glycogen synthase kinase-3β (GSK-3β) and protein kinase A (PKA) after TBI, and by that CGS21680 (A2AR agonist) exacerbated okadaic acid-induced tau hyperphosphorylation in cultured primary hippocampal neurons. Lastly, CGS21680-induced neuronal tau hyperphosphorylation and axonal injury were effectively alleviated by individual treatments with ZM241385 (A2AR antagonist), H89 (PKA antagonist) and SB216763 (GSK-3β antagonist), or by the combined treatment with H89 and SB216763. Our findings suggest a novel mechanism whereby A2AR activation triggers cognitive dysfunction by increasing the phosphorylation level of tau protein after TBI and suggest a promising therapeutic and prophylactic strategy by targeting aberrant A2AR signaling via tau phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Plasma glutamate-modulated interaction of A2AR and mGluR5 on BMDCs aggravates traumatic brain injury-induced acute lung injury.J Exp Med. 2013 Apr 8;210(4):839-51. doi: 10.1084/jem.20122196. Epub 2013 Mar 11. J Exp Med. 2013. PMID: 23478188 Free PMC article. Clinical Trial.
-
Impaired autophagic flux is associated with the severity of trauma and the role of A2AR in brain cells after traumatic brain injury.Cell Death Dis. 2018 Feb 14;9(2):252. doi: 10.1038/s41419-018-0316-4. Cell Death Dis. 2018. PMID: 29449536 Free PMC article.
-
Reduction in Blood Glutamate Levels Combined With the Genetic Inactivation of A2AR Significantly Alleviate Traumatic Brain Injury-Induced Acute Lung Injury.Shock. 2019 Apr;51(4):502-510. doi: 10.1097/SHK.0000000000001170. Shock. 2019. PMID: 29688987
-
Targeting the adenosine A2A receptor for neuroprotection and cognitive improvement in traumatic brain injury and Parkinson's disease.Chin J Traumatol. 2024 May;27(3):125-133. doi: 10.1016/j.cjtee.2023.08.003. Epub 2023 Aug 25. Chin J Traumatol. 2024. PMID: 37679245 Free PMC article. Review.
-
Role of microtubule-associated protein tau phosphorylation in Alzheimer's disease.J Huazhong Univ Sci Technolog Med Sci. 2017 Jun;37(3):307-312. doi: 10.1007/s11596-017-1732-x. Epub 2017 Jun 6. J Huazhong Univ Sci Technolog Med Sci. 2017. PMID: 28585125 Review.
Cited by
-
PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers.Cell Death Dis. 2020 Sep 24;11(9):797. doi: 10.1038/s41419-020-02998-6. Cell Death Dis. 2020. PMID: 32973135 Free PMC article. Review.
-
The Effect of Adenosine Signaling on Memory Impairment Induced by Pentylenetetrazole in Zebrafish.Neurochem Res. 2023 Jun;48(6):1889-1899. doi: 10.1007/s11064-023-03867-2. Epub 2023 Feb 2. Neurochem Res. 2023. PMID: 36729312
-
Chd8 Rescued TBI-Induced Neurological Deficits by Suppressing Apoptosis and Autophagy Via Wnt Signaling Pathway.Cell Mol Neurobiol. 2020 Oct;40(7):1165-1184. doi: 10.1007/s10571-020-00806-5. Epub 2020 Feb 7. Cell Mol Neurobiol. 2020. PMID: 32034634 Free PMC article.
-
Reactive Astrocytes as Drug Target in Alzheimer's Disease.Biomed Res Int. 2018 May 14;2018:4160247. doi: 10.1155/2018/4160247. eCollection 2018. Biomed Res Int. 2018. PMID: 29888263 Free PMC article. Review.
-
Potential Neuroprotective Mechanisms of Methamphetamine Treatment in Traumatic Brain Injury Defined by Large-Scale IonStar-Based Quantitative Proteomics.Int J Mol Sci. 2021 Feb 24;22(5):2246. doi: 10.3390/ijms22052246. Int J Mol Sci. 2021. PMID: 33668155 Free PMC article.
References
-
- Theeler BJ, Erickson JC. Posttraumatic headache in military personnel and veterans of the Iraq and Afghanistan conflicts. Curr Treat Options Neurol 2012; 14: 36–49. - PubMed
-
- Moradi AR, Abdi A, Fathi-Ashtiani A, Dalgleish T, Jobson L. Over general autobiographical memory recollection in Iranian combat veterans with posttraumatic stress disorder. Behav Res Ther 2012; 50: 435–441. - PubMed
-
- Bryan CJ, Clemans TA. Repetitive traumatic brain injury, psychological symptoms, and suicide risk in a clinical sample of deployed military personnel. JAMA Psychiatry 2013; 70: 686–691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

