Podocyte-specific soluble epoxide hydrolase deficiency in mice attenuates acute kidney injury
- PMID: 28485854
- PMCID: PMC5515292
- DOI: 10.1111/febs.14100
Podocyte-specific soluble epoxide hydrolase deficiency in mice attenuates acute kidney injury
Erratum in
-
Corrigendum.FEBS J. 2018 Feb;285(3):629-632. doi: 10.1111/febs.14361. Epub 2018 Jan 16. FEBS J. 2018. PMID: 29399996 No abstract available.
Abstract
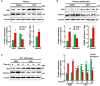
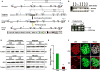
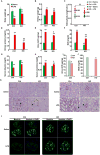
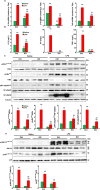
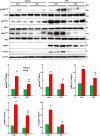
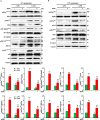
Podocytes play an important role in maintaining glomerular function, and podocyte injury is a significant component in the pathogenesis of proteinuria. Soluble epoxide hydrolase (sEH) is a cytosolic enzyme whose genetic deficiency and pharmacological inhibition have beneficial effects on renal function, but its role in podocytes remains unexplored. The objective of this study was to investigate the contribution of sEH in podocytes to lipopolysaccharide (LPS)-induced kidney injury. We report increased sEH transcript and protein expression in murine podocytes upon LPS challenge. To determine the function of sEH in podocytes in vivo we generated podocyte-specific sEH-deficient (pod-sEHKO) mice. Following LPS challenge, podocyte sEH-deficient mice exhibited lower kidney injury, proteinuria, and blood urea nitrogen concentrations than controls suggestive of preserved renal function. Also, renal mRNA and serum concentrations of inflammatory cytokines IL-6, IL-1β, and TNFα were significantly lower in LPS-treated pod-sEHKO than control mice. Moreover, podocyte sEH deficiency was associated with decreased LPS-induced NF-κB and MAPK activation and attenuated endoplasmic reticulum stress. Furthermore, the protective effects of podocyte sEH deficiency in vivo were recapitulated in E11 murine podocytes treated with a selective sEH pharmacological inhibitor. Altogether, these findings identify sEH in podocytes as a contributor to signaling events in acute renal injury and suggest that sEH inhibition may be of therapeutic value in proteinuria.
Enzymes: Soluble epoxide hydrolase: EC 3.3.2.10.
Keywords: knockout mice; podocyte; proteinuria; soluble epoxide hydrolase.
© 2017 Federation of European Biochemical Societies.
Figures
Similar articles
-
Soluble Epoxide Hydrolase Inhibition Attenuates Proteinuria by Alleviating Renal Inflammation and Podocyte Injuries in Adriamycin-Induced Nephropathy.Int J Mol Sci. 2024 Oct 2;25(19):10629. doi: 10.3390/ijms251910629. Int J Mol Sci. 2024. PMID: 39408958 Free PMC article.
-
Soluble epoxide hydrolase in podocytes is a significant contributor to renal function under hyperglycemia.Biochim Biophys Acta Gen Subj. 2017 Nov;1861(11 Pt A):2758-2765. doi: 10.1016/j.bbagen.2017.07.021. Epub 2017 Jul 27. Biochim Biophys Acta Gen Subj. 2017. PMID: 28757338 Free PMC article.
-
Protein tyrosine phosphatase Shp2 deficiency in podocytes attenuates lipopolysaccharide-induced proteinuria.Sci Rep. 2017 Mar 28;7(1):461. doi: 10.1038/s41598-017-00564-3. Sci Rep. 2017. PMID: 28352079 Free PMC article.
-
Soluble epoxide hydrolase deficiency or inhibition enhances murine hypoxic pulmonary vasoconstriction after lipopolysaccharide challenge.Am J Physiol Lung Cell Mol Physiol. 2016 Dec 1;311(6):L1213-L1221. doi: 10.1152/ajplung.00394.2016. Epub 2016 Nov 4. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27815261 Free PMC article.
-
Meloxicam fails to augment the reno-protective effects of soluble epoxide hydrolase inhibition in streptozotocin-induced diabetic rats via increased 20-HETE levels.Prostaglandins Other Lipid Mediat. 2017 Sep;132:3-11. doi: 10.1016/j.prostaglandins.2016.08.004. Epub 2016 Sep 3. Prostaglandins Other Lipid Mediat. 2017. PMID: 27596333 Review.
Cited by
-
Soluble Epoxide Hydrolase Inhibition Attenuates Proteinuria by Alleviating Renal Inflammation and Podocyte Injuries in Adriamycin-Induced Nephropathy.Int J Mol Sci. 2024 Oct 2;25(19):10629. doi: 10.3390/ijms251910629. Int J Mol Sci. 2024. PMID: 39408958 Free PMC article.
-
Repositioning of Quinazolinedione-Based Compounds on Soluble Epoxide Hydrolase (sEH) through 3D Structure-Based Pharmacophore Model-Driven Investigation.Molecules. 2022 Jun 16;27(12):3866. doi: 10.3390/molecules27123866. Molecules. 2022. PMID: 35744994 Free PMC article.
-
Chow fed UC Davis strain female Lepr fatty Zucker rats exhibit mild glucose intolerance, hypertriglyceridemia, and increased urine volume, all reduced by a Brown Norway strain chromosome 1 congenic donor region.PLoS One. 2017 Dec 6;12(12):e0188175. doi: 10.1371/journal.pone.0188175. eCollection 2017. PLoS One. 2017. PMID: 29211750 Free PMC article.
-
Downregulation of miR-29b-3p aggravates podocyte injury by targeting HDAC4 in LPS-induced acute kidney injury.Kaohsiung J Med Sci. 2021 Dec;37(12):1069-1076. doi: 10.1002/kjm2.12431. Epub 2021 Aug 9. Kaohsiung J Med Sci. 2021. PMID: 34369661 Free PMC article.
-
Long Non-Coding RNA Nuclear Paraspeckle Assembly Transcript 1 (NEAT1)Relieves Sepsis-Induced Kidney Injury and Lipopolysaccharide (LPS)-Induced Inflammation in HK-2 Cells.Med Sci Monit. 2020 Jul 29;26:e921906. doi: 10.12659/MSM.921906. Med Sci Monit. 2020. PMID: 32724027 Free PMC article.
References
-
- Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clinical and experimental nephrology. 2003;7:255–9. - PubMed
-
- Komenda P, Rigatto C, Tangri N. Estimated glomerular filtration rate and albuminuria: diagnosis, staging, and prognosis. Current opinion in nephrology and hypertension. 2014;23:251–7. - PubMed
-
- Gill SS, Hammock BD. Distribution and properties of a mammalian soluble epoxide hydrase. Biochemical pharmacology. 1980;29:389–95. - PubMed
-
- Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circulation research. 2000;87:992–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

