Use of Nanoparticle Contrast Agents for Cell Tracking with Computed Tomography
- PMID: 28485976
- PMCID: PMC5481820
- DOI: 10.1021/acs.bioconjchem.7b00194
Use of Nanoparticle Contrast Agents for Cell Tracking with Computed Tomography
Abstract
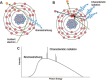
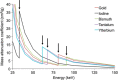
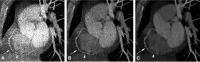
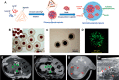
Efforts to develop novel cell-based therapies originated with the first bone marrow transplant on a leukemia patient in 1956. Preclinical and clinical examples of cell-based treatment strategies have shown promising results across many disciplines in medicine, with recent advances in immune cell therapies for cancer producing remarkable response rates, even in patients with multiple treatment failures. However, cell-based therapies suffer from inconsistent outcomes, motivating the search for tools that allow monitoring of cell delivery and behavior in vivo. Noninvasive cell imaging techniques, also known as cell tracking, have been developed to address this issue. These tools can allow real-time, quantitative, and long-term monitoring of transplanted cells in the recipient, providing insight on cell migration, distribution, viability, differentiation, and fate, all of which play crucial roles in treatment efficacy. Understanding these parameters allows the optimization of cell choice, delivery route, and dosage for therapy and advances cell-based therapy for specific clinical uses. To date, most cell tracking work has centered on imaging modalities such as MRI, radionuclide imaging, and optical imaging. However, X-ray computed tomography (CT) is an emerging method for cell tracking that has several strengths such as high spatial and temporal resolution, and excellent quantitative capabilities. The advantages of CT for cell tracking are enhanced by its wide availability and cost effectiveness, allowing CT to become one of the most popular clinical imaging modalities and a key asset in disease diagnosis. In this review, we will discuss recent advances in cell tracking methods using X-ray CT in various applications, in addition to predictions on how the field will progress.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


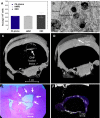

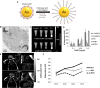
References
-
- OECD, Computed tomography (CT) exams (indicator) 2017.
-
- Cheheltani R.; Ezzibdeh R. M.; Chhour P.; Pulaparthi K.; Kim J.; Jurcova M.; Hsu J. C.; Blundell C.; Litt H. I.; Ferrari V. A.; et al. (2016) Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 102, 87–97. 10.1016/j.biomaterials.2016.06.015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

