Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy
- PMID: 28486109
- PMCID: PMC5650691
- DOI: 10.1016/j.ccell.2017.04.003
Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy
Abstract
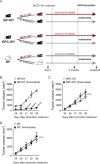
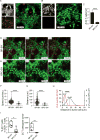
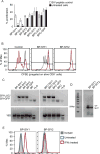
Effector T cells have the capability of recognizing and killing cancer cells. However, whether tumors can become immune resistant through exclusion of effector T cells from the tumor microenvironment is not known. By using a tumor model resembling non-T cell-inflamed human tumors, we assessed whether adoptive T cell transfer might overcome failed spontaneous priming. Flow cytometric assays combined with intra-vital imaging indicated failed trafficking of effector T cells into tumors. Mechanistically, this was due to the absence of CXCL9/10, which we found to be produced by CD103+ dendritic cells (DCs) in T cell-inflamed tumors. Our data indicate that lack of CD103+ DCs within the tumor microenvironment dominantly resists the effector phase of an anti-tumor T cell response, contributing to immune escape.
Keywords: T cell-inflamed tumor microenvironment; adoptive T cell transfer; immune escape; immunotherapy resistance; non-T cell-inflamed tumor microenvironment.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
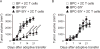



Comment in
-
Tumor Microenvironment: No Effector T Cells without Dendritic Cells.Cancer Cell. 2017 May 8;31(5):614-615. doi: 10.1016/j.ccell.2017.04.007. Cancer Cell. 2017. PMID: 28486102
References
-
- Bosenberg M, Muthusamy V, Curley DP, Wang Z, Hobbs C, Nelson B, Nogueira C, Horner JW, 2nd, Depinho R, Chin L. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis. 2006;44:262–267. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

