Strong upregulation of inflammatory genes accompanies photoreceptor demise in canine models of retinal degeneration
- PMID: 28486508
- PMCID: PMC5423635
- DOI: 10.1371/journal.pone.0177224
Strong upregulation of inflammatory genes accompanies photoreceptor demise in canine models of retinal degeneration
Abstract
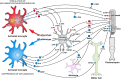
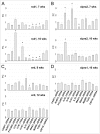
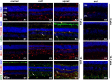
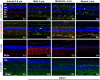
We have analyzed the complex pattern of the inflammatory response in early-onset canine models of human retinitis pigmentosa, rcd1, xlpra2 and erd, as well as late-onset xlpra1, in comparative manner. The time course of immune response genes and proteins expression was examined along the timeline of photoreceptors degeneration. Gene expression analysis of the early-onset models prior to and after the peak of photoreceptors death identified the involvement of multiple immune response genes including those encoding constituents of the NLRP3 inflammasome, its substrates, pro-IL1B, pro-IL18, and common components of IL1B, IL18 and TLR4 pathways. Out of two activated caspase-1 cleavage products, IL1B and IL18, only IL1B was detected in rcd1 and xlpra2 while precursor IL18 remained unprocessed in the same protein extract highlighting prominence of IL1B pathway. An overall immune response was most prominent in rcd1 followed by xlpra2 and least prominent in erd. Noticeably, in rcd1 and xlpra2, but not in erd, early induction of the immune response was accompanied by sustained intraretinal migration and activation of retinal microglia. Lastly, delayed activation of the anti-inflammatory factors in all early-onset models was insufficient to counterbalance rapidly progressing inflammation. In contrast to early-onset models, in late-onset xlpra1 retinas a subset of the pro-inflammatory genes was highly upregulated long before any disease-related structural changes occurred, but was counterbalanced by an adequate anti-inflammatory response. Results point out to upregulated immune response accompanying disease progression in animal models of retinal degeneration, and to potential benefits of early anti-inflammatory therapy.
Conflict of interest statement
Figures

Similar articles
-
Altered miRNA expression in canine retinas during normal development and in models of retinal degeneration.BMC Genomics. 2014 Mar 1;15(1):172. doi: 10.1186/1471-2164-15-172. BMC Genomics. 2014. PMID: 24581223 Free PMC article.
-
CREB1/ATF1 activation in photoreceptor degeneration and protection.Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5355-63. doi: 10.1167/iovs.09-3741. Epub 2009 Jul 30. Invest Ophthalmol Vis Sci. 2009. PMID: 19643965 Free PMC article.
-
Age-dependent disease expression determines remodeling of the retinal mosaic in carriers of RPGR exon ORF15 mutations.Invest Ophthalmol Vis Sci. 2009 Aug;50(8):3985-95. doi: 10.1167/iovs.08-3364. Epub 2009 Feb 28. Invest Ophthalmol Vis Sci. 2009. PMID: 19255154 Free PMC article.
-
Programmed cell death in retinal degeneration: targeting apoptosis in photoreceptors as potential therapy for retinal degeneration.Cell Cycle. 2007 Mar 15;6(6):652-5. doi: 10.4161/cc.6.6.4029. Epub 2007 Mar 17. Cell Cycle. 2007. PMID: 17374995 Review.
-
What Can Pharmacological Models of Retinal Degeneration Tell Us?Curr Mol Med. 2017;17(2):100-107. doi: 10.2174/1566524017666170331162048. Curr Mol Med. 2017. PMID: 28429669 Review.
Cited by
-
Identification of circular RNAs hosted by the RPGR ORF15 genomic locus.RNA Biol. 2023 Jan;20(1):31-47. doi: 10.1080/15476286.2022.2159165. RNA Biol. 2023. PMID: 36593651 Free PMC article.
-
miR-9-5p regulates immunometabolic and epigenetic pathways in β-glucan-trained immunity via IDH3α.JCI Insight. 2021 May 10;6(9):e144260. doi: 10.1172/jci.insight.144260. JCI Insight. 2021. PMID: 33986196 Free PMC article.
-
Myeloid masquerade: Microglial transcriptional signatures in retinal development and disease.Front Cell Neurosci. 2023 Jan 26;17:1106547. doi: 10.3389/fncel.2023.1106547. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36779012 Free PMC article. Review.
-
Endoplasmic reticulum stress: New insights into the pathogenesis and treatment of retinal degenerative diseases.Prog Retin Eye Res. 2020 Nov;79:100860. doi: 10.1016/j.preteyeres.2020.100860. Epub 2020 Apr 6. Prog Retin Eye Res. 2020. PMID: 32272207 Free PMC article. Review.
-
Retinal Inflammation, Cell Death and Inherited Retinal Dystrophies.Int J Mol Sci. 2021 Feb 20;22(4):2096. doi: 10.3390/ijms22042096. Int J Mol Sci. 2021. PMID: 33672611 Free PMC article. Review.
References
-
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76: 463–471. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

