Immortalization of chicken preadipocytes by retroviral transduction of chicken TERT and TR
- PMID: 28486516
- PMCID: PMC5423695
- DOI: 10.1371/journal.pone.0177348
Immortalization of chicken preadipocytes by retroviral transduction of chicken TERT and TR
Abstract
The chicken is an important agricultural animal and model for developmental biology, immunology and virology. Excess fat accumulation continues to be a serious problem for the chicken industry. However, chicken adipogenesis and obesity have not been well investigated, because no chicken preadipocyte cell lines have been generated thus far. Here, we successfully generated two immortalized chicken preadipocyte cell lines through transduction of either chicken telomerase reverse transcriptase (chTERT) alone or in combination with chicken telomerase RNA (chTR). Both of these cell lines have survived >100 population doublings in vitro, display high telomerase activity and have no sign of replicative senescence. Similar to primary chicken preadipocytes, these two cell lines display a fibroblast-like morphology, retain the capacity to differentiate into adipocytes, and do not display any signs of malignant transformation. Isoenzyme analysis and PCR-based analysis confirmed that these two cell lines are of chicken origin and are free from inter-species contamination. To our knowledge, this is the first report demonstrating the generation of immortal chicken cells by introduction of chTERT and chTR. Our established chicken preadipocyte cell lines show great promise as an in vitro model for the investigation of chicken adipogenesis, lipid metabolism, and obesity and its related diseases, and our results also provide clues for immortalizing other avian cell types.
Conflict of interest statement
Figures


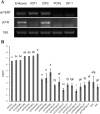
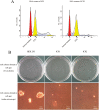



References
-
- Ji B, Ernest B, Gooding JR, Das S, Saxton AM, Simon J, et al. Transcriptomic and metabolomic profiling of chicken adipose tissue in response to insulin neutralization and fasting. BMC Genomics. 2012;13: 441 doi: 10.1186/1471-2164-13-441 - DOI - PMC - PubMed
-
- Simon J. Chicken as a useful species for the comprehension of insulin action. Crit Rev Poultry Biol. 1989;2: 121–148.
-
- Akiba Y, Chida Y, Takahashi T, Ohtomo Y, Sato K, Takahashi K. Persistent hypoglycemia induced by continuous insulin infusion in broiler chickens. Br Poult Sci. 1999;40: 701–705. doi: 10.1080/00071669987124 - DOI - PubMed
-
- Tokushima Y, Sulistiyanto B, Takahashi K, Akiba Y. Insulin-glucose interactions characterised in newly hatched broiler chicks. Br Poult Sci. 2003;44: 746–751. doi: 10.1080/00071660310001645758 - DOI - PubMed
-
- Dupont J, Metayer-Coustard S, Ji B, Rame C, Gespach C, Voy B, et al. Characterization of major elements of insulin signaling cascade in chicken adipose tissue: apparent insulin refractoriness. Gen Comp Endocrinol. 2012;176: 86–93. doi: 10.1016/j.ygcen.2011.12.030 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

