Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis
- PMID: 28486544
- PMCID: PMC5423631
- DOI: 10.1371/journal.pone.0177113
Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis
Abstract
Background: Routine immunisation with pneumococcal conjugate vaccines (PCV7/10/13) has reduced invasive pneumococcal disease (IPD) due to vaccine serotypes significantly. However, an increase in disease due to non-vaccine types, or serotype replacement, has been observed. Serotypes' individual contributions to IPD play a critical role in determining the overall effects of PCVs. This study examines the distribution of pneumococcal serotypes in children to identify leading serotypes associated with IPD post-PCV introduction.
Methods: A systematic search was performed to identify studies and surveillance reports (published between 2000 and December 2015) of pneumococcal serotypes causing childhood IPD post-PCV introduction. Serotype data were differentiated based on the PCV administered during the study period: PCV7 or higher valent PCVs (PCV10 or PCV13). Meta-analysis was conducted to estimate the proportional contributions of the most frequent serotypes in childhood IPD in each period.
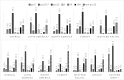
Results: We identified 68 studies reporting serotype data among IPD cases in children. We analysed data from 38 studies (14 countries) where PCV7 was administered and 20 (24 countries) where PCV10 or PCV13 have been introduced. Studies reported early and late periods of PCV7 administration (range: 2001∓13). In these settings, serotype 19A was the most predominant cause of childhood IPD, accounting for 21.8% (95%CI 18.6∓25.6) of cases. In countries that have introduced higher valent PCVs, study periods were largely representative of the transition and early years of PCV10 or PCV13. In these studies, the overall serotype-specific contribution of 19A was lower (14.2% 95%CI 11.1∓18.3). Overall, non-PCV13 serotypes contributed to 42.2% (95%CI 36.1∓49.5%) of childhood IPD cases. However, regional differences were noted (57.8% in North America, 71.9% in Europe, 45.9% in Western Pacific, 28.5% in Latin America, 42.7% in one African country, and 9.2% in one Eastern Mediterranean country). Predominant non-PCV13 serotypes overall were 22F, 12F, 33F, 24F, 15C, 15B, 23B, 10A, and 38 (descending order), but their rank order varied by region.
Conclusion: Childhood IPD is associated with a wide number of serotypes. In the early years after introduction of higher valent PCVs, non-PCV13 types caused a considerable proportion of childhood IPD. Serotype data, particularly from resource-limited countries with high burden of IPD, are needed to assess the importance of serotypes in different settings. The geographic diversity of pneumococcal serotypes highlights the importance of continued surveillance to guide vaccine design and recommendations.
Conflict of interest statement
Figures
References
-
- World Health Organization. Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008 2008 [cited 2016 28 March]. http://www.who.int/immunization/monitoring_surveillance/burden/estimates....
-
- World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2015 global summary 2015. http://apps.who.int/immunization_monitoring/globalsummary/schedules.
-
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. Vaccine Information Management System (VIMS) Global Vaccine Introduction Report 2015. www.jhsph.edu/ivac/vims.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical