Non-Redfield, nutrient synergy and flexible internal elemental stoichiometry in a marine bacterium
- PMID: 28486660
- PMCID: PMC5458051
- DOI: 10.1093/femsec/fix059
Non-Redfield, nutrient synergy and flexible internal elemental stoichiometry in a marine bacterium
Abstract
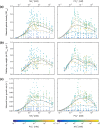
The stoichiometric constraints of algal growth are well understood, whereas there is less knowledge for heterotrophic bacterioplankton. Growth of the marine bacterium Phaeobacter inhibens DSM 17395, belonging to the globally distributed Roseobacter group, was studied across a wide concentration range of NH4+ and PO43-. The unique dataset covers 415 different concentration pairs, corresponding to 207 different molar N:P ratios (from 10-2 to 105). Maximal growth (by growth rate and biomass yield) was observed within a restricted concentration range at N:P ratios (∼50-120) markedly above Redfield. Experimentally determined growth parameters deviated to a large part from model predictions based on Liebig's law of the minimum, thus implicating synergistic co-limitation due to biochemical dependence of resources. Internal elemental ratios of P. inhibens varied with external nutrient supply within physiological constraints, thus adding to the growing evidence that aquatic bacteria can be flexible in their internal elemental composition. Taken together, the findings reported here revealed that P. inhibens is well adapted to fluctuating availability of inorganic N and P, expected to occur in its natural habitat (e.g. colonized algae, coastal areas). Moreover, this study suggests that elemental variability in bacterioplankton needs to be considered in the ecological stoichiometry of the oceans.
Keywords: Liebig limitation; N:P ratio; Phaeobacter inhibens DSM 17395; Redfield; ecological stoichiometry; growth physiology.
© FEMS 2017.
Figures





References
-
- Allen MM. Cyanobacterial cell inclusions. Ann Rev Microbiol 1984;38:1–25. - PubMed
-
- Azam F, Malfatti F. Microbial structuring of marine ecosystems. Nat Rev Microbiol 2007;5:782–91. - PubMed
-
- Beckon WN, Parkins C, Maximovich A et al. . A general approach to modeling biphasic relationships. Environ Sci Technol 2008;42:1308–14. - PubMed
-
- Bidle KD, Azam F. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature 1999;397:508–12.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

