Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread
- PMID: 28486838
- PMCID: PMC5665495
- DOI: 10.1089/AID.2017.0004
Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread
Abstract
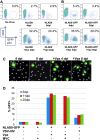
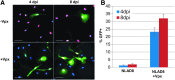
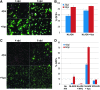
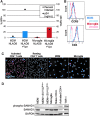
Macrophages are a target of human immunodeficiency virus type 1 (HIV-1) infection and may serve as an important reservoir of the virus in the body, particularly after depletion of CD4+ T cells in HIV/AIDS. Recently, sterile alpha motif and histidine/aspartic acid domain-containing protein 1 (SAMHD1) was identified as the major restriction factor of HIV-1 infection in myeloid cells. SAMHD1 is targeted for proteolytic degradation by Vpx, a viral protein encoded by HIV-2 and many simian immunodeficiency viruses but not HIV-1. In this study, we assessed SAMHD1 restriction in in vitro differentiated macrophages and in freshly isolated macrophages from the lungs, abdomen, and brain. We found that infection and spread in in vitro cultured monocyte-derived macrophages were highly limited and that Vpx largely relieved the restriction to initial infection, as expected. We observed nearly identical infection and restriction profiles in freshly isolated peripheral blood monocytes, as well as lung (alveolar) and abdominal (peritoneal) macrophages. In contrast, under the same infection conditions, primary brain macrophages (microglia) were highly susceptible to HIV-1 infection despite levels of endogenous SAMHD1 comparable to the other macrophage populations. Addition of Vpx further increased HIV-1 infection under conditions of limiting virus input, and viral spread was robust whether or not SAMHD1 was depleted. These results suggest that HIV-1 infection of peripherally circulating macrophages is effectively restricted by SAMHD1; however, microglia are highly susceptible to infection despite SAMHD1 expression. These data may explain the long-standing observation that HIV-1 infection is often detected in macrophages in the brain, but seldom in other tissues of the body.
Keywords: HIV-1; macrophage; microglia.
Conflict of interest statement
No competing financial interests exist.
Figures

References
-
- Groot F, Welsch S, Sattentau QJ: Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 2008;111:4660–4663 - PubMed
-
- Igarashi T, et al. : Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc Natl Acad Sci U S A 2001;98:658–663 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

