A role for cathepsin Z in neuroinflammation provides mechanistic support for an epigenetic risk factor in multiple sclerosis
- PMID: 28486971
- PMCID: PMC5424360
- DOI: 10.1186/s12974-017-0874-x
A role for cathepsin Z in neuroinflammation provides mechanistic support for an epigenetic risk factor in multiple sclerosis
Abstract
Background: Hypomethylation of the cathepsin Z locus has been proposed as an epigenetic risk factor for multiple sclerosis (MS). Cathepsin Z is a unique lysosomal cysteine cathepsin expressed primarily by antigen presenting cells. While cathepsin Z expression has been associated with neuroinflammatory disorders, a role for cathepsin Z in mediating neuroinflammation has not been previously established.
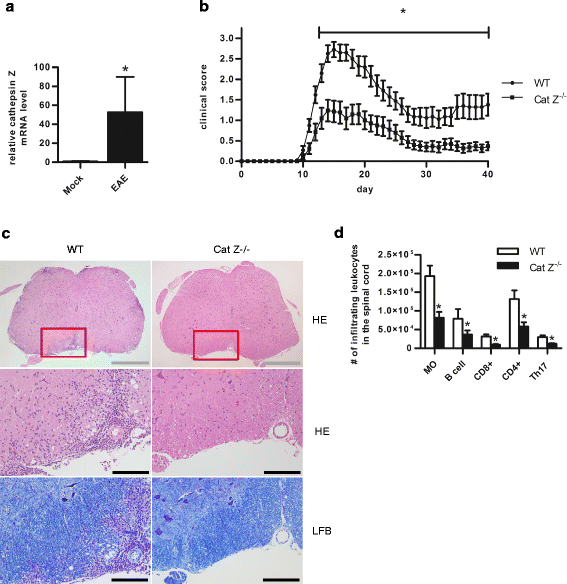
Methods: Experimental autoimmune encephalomyelitis (EAE) was induced in both wildtype mice and mice deficient in cathepsin Z. The effects of cathepsin Z-deficiency on the processing and presentation of the autoantigen myelin oligodendrocyte glycoprotein, and on the production of IL-1β and IL-18 were determined in vitro from cells derived from wildtype and cathepsin Z-deficient mice. The effects of cathepsin Z-deficiency on CD4+ T cell activation, migration, and infiltration to the CNS were determined in vivo. Statistical analyses of parametric data were performed by one-way ANOVA followed by Tukey post-hoc tests, or by an unpaired Student's t test. EAE clinical scoring was analyzed using the Mann-Whitney U test.
Results: We showed that mice deficient in cathepsin Z have reduced neuroinflammation and dramatically lowered circulating levels of IL-1β during EAE. Deficiency in cathepsin Z did not impact either the processing or the presentation of MOG, or MOG- specific CD4+ T cell activation and trafficking. Consistently, we found that cathepsin Z-deficiency reduced the efficiency of antigen presenting cells to secrete IL-1β, which in turn reduced the ability of mice to generate Th17 responses-critical steps in the pathogenesis of EAE and MS.
Conclusion: Together, these data support a novel role for cathepsin Z in the propagation of IL-1β-driven neuroinflammation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

