Reduced-cost Chlamydia trachomatis-specific multiplex real-time PCR diagnostic assay evaluated for ocular swabs and use by trachoma research programmes
- PMID: 28487054
- PMCID: PMC5496587
- DOI: 10.1016/j.mimet.2017.04.010
Reduced-cost Chlamydia trachomatis-specific multiplex real-time PCR diagnostic assay evaluated for ocular swabs and use by trachoma research programmes
Abstract
Introduction: Trachoma, caused by the intracellular bacterium Chlamydia trachomatis (Ct), is the leading infectious cause of preventable blindness. Many commercial platforms are available that provide highly sensitive and specific detection of Ct DNA. However, the majority of these commercial platforms are inaccessible for population-level surveys in resource-limited settings typical to trachoma control programmes. We developed two low-cost quantitative PCR (qPCR) tests for Ct using readily available reagents on standard real-time thermocyclers.
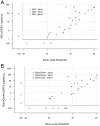
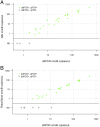
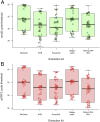
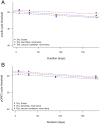
Methods: Each multiplex qPCR test targets one genomic and one plasmid Ct target in addition to an endogenous positive control for Homo sapiens DNA. The quantitative performance of the qPCR assays in clinical samples was determined by comparison to a previously evaluated droplet digital PCR (ddPCR) test. The diagnostic performance of the qPCR assays were evaluated against a commercial assay (artus C. trachomatis Plus RG PCR, Qiagen) using molecular diagnostics quality control standards and clinical samples. We examined the yield of Ct DNA prepared from five different DNA extraction kits and a cold chain-free dry-sample preservation method using swabs spiked with fixed concentrations of human and Ct DNA.
Results: The qPCR assay was highly reproducible (Ct plasmid and genomic targets mean total coefficients of variance 41.5% and 48.3%, respectively). The assay detected 8/8 core specimens upon testing of a quality control panel and performed well in comparison to commercially marketed comparator test (sensitivity and specificity>90%). Optimal extraction and sample preservation methods for research applications were identified.
Conclusion: We describe a pipeline from collection to diagnosis providing the most efficient sample preservation and extraction with significant per test cost savings over a commercial qPCR diagnostic assay. The assay and its evaluation should allow control programs wishing to conduct independent research within the context of trachoma control, access to an affordable test with defined performance characteristics.
Keywords: Chlamydia trachomatis; Diagnosis; Quantitative PCR; Trachoma.
Copyright © 2017. Published by Elsevier B.V.
Figures

Similar articles
-
Evaluation of a Chlamydia trachomatis-specific, commercial, real-time PCR for use with ocular swabs.Parasit Vectors. 2018 Feb 20;11(1):102. doi: 10.1186/s13071-018-2686-y. Parasit Vectors. 2018. PMID: 29463279 Free PMC article.
-
Development, validation and testing costs of an in-house real-time PCR assay for the detection of Chlamydia trachomatis.J Med Microbiol. 2017 Mar;66(3):312-317. doi: 10.1099/jmm.0.000443. Epub 2017 Mar 20. J Med Microbiol. 2017. PMID: 28141509
-
A multiplex PCR assay for the simultaneous detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.Exp Mol Pathol. 2015 Apr;98(2):214-8. doi: 10.1016/j.yexmp.2015.01.011. Epub 2015 Jan 13. Exp Mol Pathol. 2015. PMID: 25595915
-
Systematic reviews of point-of-care tests for the diagnosis of urogenital Chlamydia trachomatis infections.Sex Transm Infect. 2017 Dec;93(S4):S22-S30. doi: 10.1136/sextrans-2016-053067. Sex Transm Infect. 2017. PMID: 29223960 Review.
-
Diagnosis and assessment of trachoma.Clin Microbiol Rev. 2004 Oct;17(4):982-1011, table of contents. doi: 10.1128/CMR.17.4.982-1011.2004. Clin Microbiol Rev. 2004. PMID: 15489358 Free PMC article. Review.
Cited by
-
Longitudinal changes in tear cytokines and antimicrobial proteins in trachomatous disease.PLoS Negl Trop Dis. 2023 Oct 20;17(10):e0011689. doi: 10.1371/journal.pntd.0011689. eCollection 2023 Oct. PLoS Negl Trop Dis. 2023. PMID: 37862368 Free PMC article.
-
Ocular immune responses, Chlamydia trachomatis infection and clinical signs of trachoma before and after azithromycin mass drug administration in a treatment naïve trachoma-endemic Tanzanian community.PLoS Negl Trop Dis. 2019 Jul 15;13(7):e0007559. doi: 10.1371/journal.pntd.0007559. eCollection 2019 Jul. PLoS Negl Trop Dis. 2019. PMID: 31306419 Free PMC article.
-
The conjunctival transcriptome in Ethiopians after trichiasis surgery: associations with the development of eyelid contour abnormalities and the effect of oral doxycycline treatment.Wellcome Open Res. 2022 Nov 11;4:130. doi: 10.12688/wellcomeopenres.15419.2. eCollection 2019. Wellcome Open Res. 2022. PMID: 37426632 Free PMC article.
-
Comparison of genovars and Chlamydia trachomatis infection loads in ocular samples from children in two distinct cohorts in Sudan and Morocco.PLoS Negl Trop Dis. 2021 Aug 9;15(8):e0009655. doi: 10.1371/journal.pntd.0009655. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34370735 Free PMC article.
-
Trachoma risk factors in Oromia Region, Ethiopia.PLoS Negl Trop Dis. 2023 Nov 7;17(11):e0011679. doi: 10.1371/journal.pntd.0011679. eCollection 2023 Nov. PLoS Negl Trop Dis. 2023. PMID: 37934731 Free PMC article.
References
-
- Bourne R.A., Stevens G.A., White R.A., Smith J.L., Flaxman S.R., Price H. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob. Health. 2013;1(6):e339–e349. - PubMed
-
- Burton M.J., Holland M.J., Faal N., Aryee E.A.N., Alexander N.D.E., Bah M. Which members of a community need antibiotics to control trachoma? Conjunctival Chlamydia trachomatis infection load in Gambian villages. Invest. Ophthalmol. Vis. Sci. 2003 Oct;44(10):4215–4222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

