Development of nanomaterials for bone-targeted drug delivery
- PMID: 28487069
- PMCID: PMC5644493
- DOI: 10.1016/j.drudis.2017.04.021
Development of nanomaterials for bone-targeted drug delivery
Abstract
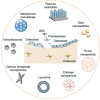
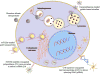
Bone is one of the major organs of the human body; it supports and protects other organs, produces blood cells, stores minerals, and regulates hormones. Therefore, disorders in bone can cause serious morbidity, complications, or mortality of patients. However, despite the significant occurrence of bone diseases, such as osteoarthritis (OA), osteoporosis (OP), non-union bone defects, bone cancer, and myeloma-related bone disease, their effective treatments remain a challenge. In this review, we highlight recent progress in the development of nanotechnology-based drug delivery for bone treatment, based on its improved delivery efficiency and safety. We summarize the most commonly used nanomaterials for bone drug delivery. We then discuss the targeting strategies of these nanomaterials to the diseased sites of bone tissue. We also highlight nanotechnology-based drug delivery to bone cells and subcellular organelles. We envision that nanotechnology-based drug delivery will serve as a powerful tool for developing treatments for currently incurable bone diseases.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



References
-
- Baglioni P, et al. Nanomaterials in art conservation. Nat. Nanotechnol. 2015;10:287–290. - PubMed
-
- Jiang W, et al. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008;3:145–150. - PubMed
-
- Hubbell JA, Chilkoti A. Nanomaterials for drug delivery. Science. 2012;337:303–305. - PubMed
-
- Ma X, et al. Future of nanotherapeutics: targeting the cellular sub-organelles. Biomaterials. 2016;97:10–21. - PubMed
-
- Lee DE, et al. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012;41:2656–2672. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

