Human LRRK2 G2019S mutation represses post-synaptic protein PSD95 and causes cognitive impairment in transgenic mice
- PMID: 28487191
- PMCID: PMC5568523
- DOI: 10.1016/j.nlm.2017.05.001
Human LRRK2 G2019S mutation represses post-synaptic protein PSD95 and causes cognitive impairment in transgenic mice
Abstract
Background: LRRK2 G2019S mutation is associated with increased kinase activity and is the most common mutation associated with late-onset PD. However, the transgenic mouse model has not recapitulated cardinal PD-related motor phenotypes. Non-motor symptoms of PD including cognitive impairments are very common and may appear earlier than the motor symptoms. The objective of this study was to determine whether human LRRK2 with G2019S mutation causes hippocampus-dependent cognitive deficits in mice.
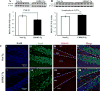
Results: Male (LRRK2-G2019S) LRRK2-Tg mice showed impairments in the early portion of the Two-day radial arm water maze acquisition trial as well as in the reversal learning on the third day. However, their performance was similar to Non-Tg controls in the probe trial. LRRK2-Tg mice also displayed impairments in the novel arm discrimination test but not in the spontaneous alternation test in Y-maze. Interestingly, there was no statistically significant locomotor impairment during any of these cognitive test, nor in the locomotor tests including open field, accelerating rotarod and pole tests. Expression of the postsynaptic protein PSD-95 but not the presynaptic protein synaptophysin was lower in hippocampal homogenates of LRRK2-Tg mice.
Conclusion: Consistent with previous reports in human LRRK2 G2019S carriers, the current data suggests that cognitive dysfunctions are present in LRRK2-Tg mice even in the absence of locomotor impairment. LRRK2 G2019S mutation represses the postsynaptic protein PSD-95 but not the presynaptic protein synaptophysin. This study also suggests that mild cognitive impairment may appear earlier than motor dysfunctions in LRRK2-G2019S mutation carriers.
Keywords: Cognitive impairment; Hippocampus; LRRK2; Learning and memory; Parkinson’s disease.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Adeosun SO, Hou X, Jiao Y, Zheng B, Henry S, Hill R, Wang JM. Allopregnanolone reinstates tyrosine hydroxylase immunoreactive neurons and motor performance in an MPTP-lesioned mouse model of Parkinson’s disease. PLoS ONE. 2012;7:e50040. http://dx.doi.org/10.1371/journal.pone.0050040. - DOI - PMC - PubMed
-
- Adeosun SO, Hou X, Zheng B, Stockmeier C, Ou X, Paul I, Wang JM. Cognitive deficits and disruption of neurogenesis in a mouse model of apolipoprotein E4 domain interaction. Journal of Biological Chemistry. 2014;289:2946–2959. http://dx.doi.org/10.1074/jbc.M113.497909. - DOI - PMC - PubMed
-
- Ahmad I, Lope-Piedrafita S, Bi X, Hicks C, Yao Y, Yu C, Erickson RP. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. Journal of Neuroscience Research. 2005;82:811–821. < http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve%db=PubMed%dop...>. - PubMed
-
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nature Protocols. 2006;1:1671–1679. http://dx.doi.org/10.1038/nprot.2006.275. - DOI - PubMed
-
- Bailey RM, Covy JP, Melrose HL, Rousseau L, Watkinson R, Knight J, Lewis J. LRRK2 phosphorylates novel tau epitopes and promotes tauopathy. Acta Neuropathologica. 2013;126:809–827. http://dx.doi.org/10.1007/s00401-013-1188-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

