XBP1 (X-Box-Binding Protein-1)-Dependent O-GlcNAcylation Is Neuroprotective in Ischemic Stroke in Young Mice and Its Impairment in Aged Mice Is Rescued by Thiamet-G
- PMID: 28487326
- PMCID: PMC5493893
- DOI: 10.1161/STROKEAHA.117.016579
XBP1 (X-Box-Binding Protein-1)-Dependent O-GlcNAcylation Is Neuroprotective in Ischemic Stroke in Young Mice and Its Impairment in Aged Mice Is Rescued by Thiamet-G
Abstract
Background and purpose: Impaired protein homeostasis induced by endoplasmic reticulum dysfunction is a key feature of a variety of age-related brain diseases including stroke. To restore endoplasmic reticulum function impaired by stress, the unfolded protein response is activated. A key unfolded protein response prosurvival pathway is controlled by the endoplasmic reticulum stress sensor (inositol-requiring enzyme-1), XBP1 (downstream X-box-binding protein-1), and O-GlcNAc (O-linked β-N-acetylglucosamine) modification of proteins (O-GlcNAcylation). Stroke impairs endoplasmic reticulum function, which activates unfolded protein response. The rationale of this study was to explore the potentials of the IRE1/XBP1/O-GlcNAc axis as a target for neuroprotection in ischemic stroke.
Methods: Mice with Xbp1 loss and gain of function in neurons were generated. Stroke was induced by transient or permanent occlusion of the middle cerebral artery in young and aged mice. Thiamet-G was used to increase O-GlcNAcylation.
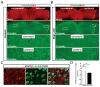
Results: Deletion of Xbp1 worsened outcome after transient and permanent middle cerebral artery occlusion. After stroke, O-GlcNAcylation was activated in neurons of the stroke penumbra in young mice, which was largely Xbp1 dependent. This activation of O-GlcNAcylation was impaired in aged mice. Pharmacological increase of O-GlcNAcylation before or after stroke improved outcome in both young and aged mice.
Conclusions: Our study indicates a critical role for the IRE1/XBP1 unfolded protein response branch in stroke outcome. O-GlcNAcylation is a prosurvival pathway that is activated in the stroke penumbra in young mice but impaired in aged mice. Boosting prosurvival pathways to counterbalance the age-related decline in the brain's self-healing capacity could be a promising strategy to improve ischemic stroke outcome in aged brains.
Keywords: O-GlcNAc; XBP1; ischemic stroke; neuroprotection; thiamet-G.
© 2017 American Heart Association, Inc.
Conflict of interest statement
Figures




References
-
- Paschen W, Frandsen A. Endoplasmic reticulum dysfunction--a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem. 2001;79:719–725. - PubMed
-
- Paschen W, Aufenberg C, Hotop S, Mengesdorf T. Transient cerebral ischemia activates processing of xbp1 messenger RNA indicative of endoplasmic reticulum stress. J Cereb Blood Flow Metab. 2003;23:449–461. - PubMed
-
- Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18:59–68. - PubMed
-
- Dickhout JG, Carlisle RE, Austin RC. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: Endoplasmic reticulum stress as a mediator of pathogenesis. Circ Res. 2011;108:629–642. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

