Ion channels and ion selectivity
- PMID: 28487397
- PMCID: PMC5544903
- DOI: 10.1042/EBC20160074
Ion channels and ion selectivity
Abstract
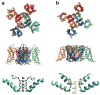
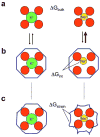
Specific macromolecular transport systems, ion channels and pumps, provide the pathways to facilitate and control the passage of ions across the lipid membrane. Ion channels provide energetically favourable passage for ions to diffuse rapidly and passively according to their electrochemical potential. Selective ion channels are essential for the excitability of biological membranes: the action potential is a transient phenomenon that reflects the rapid opening and closing of voltage-dependent Na+-selective and K+-selective channels. One of the most critical functional aspects of K+ channels is their ability to remain highly selective for K+ over Na+ while allowing high-throughput ion conduction at a rate close to the diffusion limit. Permeation through the K+ channel selectivity filter is believed to proceed as a 'knockon' mechanism, in which 2-3 K+ ions interspersed by water molecules move in a single file. Permeation through the comparatively wider and less selective Na+ channels also proceeds via a loosely coupled knockon mechanism, although the ions do not need to be fully dehydrated. While simple structural concepts are often invoked to rationalize the mechanism of ion selectivity, a deeper analysis shows that subtle effects play an important role in these flexible dynamical structures.
Keywords: free energy; hydration; molecular dynamics; solvation.
© 2017 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Figures
References
-
- Allen TW, Bliznyuk A, Rendell AP, Kyucak S, Chung SH. The potassium channel: Structure, selectivity and diffusion. J Chem Phys. 2000;112:8191–8204.
-
- Åqvist J, V, Luzhkov B. Ion permeation mechanism of the potassium channel. Nature. 2000;404:881–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

