Flavodiiron Proteins Promote Fast and Transient O2 Photoreduction in Chlamydomonas
- PMID: 28487478
- PMCID: PMC5490913
- DOI: 10.1104/pp.17.00421
Flavodiiron Proteins Promote Fast and Transient O2 Photoreduction in Chlamydomonas
Abstract
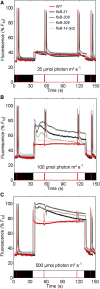
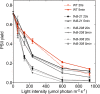
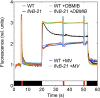
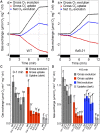
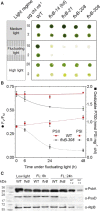
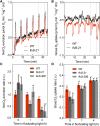
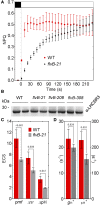
During oxygenic photosynthesis, the reducing power generated by light energy conversion is mainly used to reduce carbon dioxide. In bacteria and archae, flavodiiron (Flv) proteins catalyze O2 or NO reduction, thus protecting cells against oxidative or nitrosative stress. These proteins are found in cyanobacteria, mosses, and microalgae, but have been lost in angiosperms. Here, we used chlorophyll fluorescence and oxygen exchange measurement using [18O]-labeled O2 and a membrane inlet mass spectrometer to characterize Chlamydomonas reinhardtii flvB insertion mutants devoid of both FlvB and FlvA proteins. We show that Flv proteins are involved in a photo-dependent electron flow to oxygen, which drives most of the photosynthetic electron flow during the induction of photosynthesis. As a consequence, the chlorophyll fluorescence patterns are strongly affected in flvB mutants during a light transient, showing a lower PSII operating yield and a slower nonphotochemical quenching induction. Photoautotrophic growth of flvB mutants was indistinguishable from the wild type under constant light, but severely impaired under fluctuating light due to PSI photo damage. Remarkably, net photosynthesis of flv mutants was higher than in the wild type during the initial hour of a fluctuating light regime, but this advantage vanished under long-term exposure, and turned into PSI photo damage, thus explaining the marked growth retardation observed in these conditions. We conclude that the C. reinhardtii Flv participates in a Mehler-like reduction of O2, which drives a large part of the photosynthetic electron flow during a light transient and is thus critical for growth under fluctuating light regimes.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures
References
-
- Allen JF. (2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci 8: 15–19 - PubMed
-
- Badger MR. (1985) Photosynthetic oxygen-exchange. Annu Rev Plant Physiol Plant Mol Biol 36: 27–53
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

