Retinal Degeneration In A Mouse Model Of CLN5 Disease Is Associated With Compromised Autophagy
- PMID: 28487519
- PMCID: PMC5431647
- DOI: 10.1038/s41598-017-01716-1
Retinal Degeneration In A Mouse Model Of CLN5 Disease Is Associated With Compromised Autophagy
Abstract
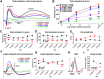
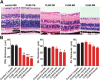
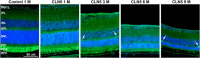
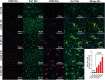
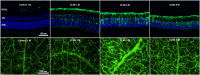
The Finnish variant of late infantile neuronal ceroid lipofuscinosis (CLN5 disease) belongs to a family of neuronal ceroid lipofuscinosis (NCLs) diseases. Vision loss is among the first clinical signs in childhood forms of NCLs. Mutations in CLN5 underlie CLN5 disease. The aim of this study was to characterize how the lack of normal functionality of the CLN5 protein affects the mouse retina. Scotopic electroretinography (ERG) showed a diminished c-wave amplitude in the CLN5 deficient mice already at 1 month of age, indicative of pathological events in the retinal pigmented epithelium. A- and b-waves showed progressive impairment later from 2 and 3 months of age onwards, respectively. Structural and immunohistochemical (IHC) analyses showed preferential damage of photoreceptors, accumulation of autofluorescent storage material, apoptosis of photoreceptors, and strong inflammation in the CLN5 deficient mice retinas. Increased levels of autophagy-associated proteins Beclin-1 and P62, and increased LC3b-II/LC3b-I ratio, were detected by Western blotting from whole retinal extracts. Photopic ERG, visual evoked potentials, IHC and cell counting indicated relatively long surviving cone photoreceptors compared to rods. In conclusion, CLN5 deficient mice develop early vision loss that reflects the condition reported in clinical childhood forms of NCLs. The vision loss in CLN5 deficient mice is primarily caused by photoreceptor degeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

