5α-Reductase Inhibitors for Treatment of Benign Prostatic Hyperplasia: A Systematic Review and Meta-Analysis
- PMID: 28487578
- PMCID: PMC5407420
- DOI: 10.4212/cjhp.v70i2.1643
5α-Reductase Inhibitors for Treatment of Benign Prostatic Hyperplasia: A Systematic Review and Meta-Analysis
Abstract
Background: Finasteride and dutasteride are competitive inhibitors of 5α-reductase enzymes and are commonly used to treat symptomatic benign prostatic hyperplasia (BPH).
Objective: To compare the efficacy and safety of finasteride and dutasteride in terms of clinically important outcomes.
Data sources: A literature search was performed using the search terms "prostatic hyperplasia", "prostatic hypertrophy", "dutasteride", "finasteride", "quality of life", "adverse drug reaction", and "mortality". The Embase, PubMed, Cochrane Central Register of Controlled Trials, International Pharmaceutical Abstracts, Cumulative Index to Nursing and Allied Health Literature, and Latin American and Caribbean Health Sciences Literature databases were searched from inception to December 2015.
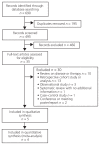
Study selection and data extraction: Randomized controlled trials, quasi-randomized trials, and systematic reviews comparing finasteride with dutasteride, either as monotherapy or in combination with α-blockers, for treatment of men with BPH were included. The outcomes of interest included need for prostate-related surgery, episodes of acute urinary retention, withdrawals due to adverse events, number of patients experiencing serious adverse events, mortality, and sexual dysfunction.
Data synthesis: Four studies involving a total of 1879 patients were included in the analysis. There were no significant differences in any of the clinically important outcomes examined: for prostate-related surgery, odds ratio (OR) 2.01 (95% confidence interval [CI] 0.18-22.24); for episodes of acute urinary retention, OR 1.47 (95% CI 0.68-3.19); for number of withdrawals due to adverse events, OR 1.10 (95% CI 0.68-1.75); for total number of patients experiencing adverse events, OR 0.94 (95% CI 0.78-1.14); for number of patients experiencing serious adverse events, OR 1.31 (95% CI 0.87-1.97); and for sexual dysfunction, OR 0.83 (95% CI 0.64-1.08).
Conclusion: There is insufficient evidence to suggest that either finasteride or dutasteride offers an advantage in efficacy or safety over the other, in terms of clinically important outcomes.
Contexte: Le finastéride et le dutastéride sont des inhibiteurs compétitifs de l’enzyme 5 alpha-réductase. Ils sont fréquemment employés comme traitement symptomatique de l’hyperplasie bénigne de la prostate (HBP).
Objectif: Comparer l’efficacité et l’innocuité du finastéride et du dutastéride en ce qui concerne les résultats thérapeutiques cliniquement importants.
Sources des données: Une recherche documentaire a été effectuée à l’aide des termes « hyperplasie de la prostate », « hypertrophie de la prostate », « dutastéride », « finastéride », « qualité de vie », « réaction indésirable aux médicaments » et « mortalité ». Les bases de données Embase, PubMed, International Pharmaceutical Abstracts, Cumulative Index to Nursing and Allied Health Literature et Latin American and Caribbean Health Sciences Literature ainsi que le Registre central Cochrane des essais comparatifs ont été interrogées pour la période allant de leur création à décembre 2015.
Sélection des études et extraction des données: Les essais comparatifs à répartition aléatoire, les essais quasi-aléatoires et les analyses systématiques qui comparent le finastéride et le dutastéride, en monothérapie ou en association avec des α-bloquants, pour le traitement de la HBP chez l’homme, ont été retenus. Parmi les résultats d’intérêt, on comptait : la nécessité de recourir à une chirurgie de la prostate, les épisodes de rétention urinaire aiguë, les retraits de l’étude pour cause d’événements indésirables, le nombre total de patients ayant subi des événements indésirables graves, la mortalité et le dysfonctionnement sexuel.
Synthèse des données: Quatre études comptant au total 1879 patients ont été retenues pour l’analyse. Aucune différence significative n’a été relevée en ce qui touche les résultats thérapeutiques cliniquement importants : la nécessité de recourir à une chirurgie de la prostate (risque relatif approché [RRA] de 2,01, intervalle de confiance [IC] à 95 % de 0,18 à 22,24), les épisodes de rétention urinaire aiguë (RRA de 1,47, IC à 95 % de 0,68 à 3,19), le nombre de retraits de l’étude pour cause d’événements indésirables (RRA de 1,10, IC à 95 % de 0,68 à 1,75), le nombre total de patients ayant subi des événements indésirables (RRA de 0,94, IC à 95 % de 0,78 à 1,14); le nombre de patients ayant subi des événements indésirables graves (RRA de 1,31, IC à 95 % de 0,87 à 1,97) et le dysfonctionnement sexuel (RRA de 0,83, IC à 95 % de 0,64 à 1,08).
Conclusion: Il n’y a pas suffisamment de données probantes pour croire que le finastéride ou le dutastéride offrent, l’un par rapport à l’autre, un avantage quant à l’efficacité ou à l’innocuité, en ce qui concerne les résultats thérapeutiques cliniquement importants.
Keywords: 5α-reductase inhibitors; benign prostatic hyperplasia; benign prostatic hypertrophy; dutasteride; finasteride.
Conflict of interest statement
Competing interests: For activities not directly related to the topic of this article, Aaron Tejani has received a grant from the College of Pharmacists of British Columbia and payment for lectures on a variety of topics from the University of British Columbia, Vancouver Island Health Authority Pharmacy Services, and various professional societies and organizations; he also serves on the UBC Therapeutics Initiative. No other competing interests were declared.
Figures


References
-
- Roehrborn C, McConnell J. Etiology, pathophysiology, epidemiology and natural history of benign prostatic hyperplasia. In: Walsh P, Retik A, Vaughan E Jr, Wein AJ, editors. Campbell’s urology. 8th ed. Philadelphia (PA): W B Saunders; 2002. pp. 1297–330.
-
- Naslund M, Regan TS, Ong C, Hogue SL. 5-Alpha reductase inhibitors in men with an enlarged prostate: an evaluation of outcomes and therapeutic alternatives. Am J Manag Care. 2008;14(5 Suppl 2):S148–53. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
