Exosomes as Carriers of Alzheimer's Amyloid-ß
- PMID: 28487629
- PMCID: PMC5403946
- DOI: 10.3389/fnins.2017.00229
Exosomes as Carriers of Alzheimer's Amyloid-ß
Abstract
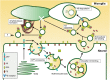
The intracerebral level of the aggregation-prone peptide, amyloid-ß (Aß), is constantly maintained by multiple clearance mechanisms, including several degradation enzymes, and brain efflux. Disruption of the clearance machinery and the resultant Aß accumulation gives rise to neurotoxic assemblies, leading to the pathogenesis of Alzheimer's disease (AD). In addition to the classic mechanisms of Aß clearance, the protein may be processed by secreted vesicles, although this possibility has not been extensively investigated. We showed that neuronal exosomes, a subtype of extracellular nanovesicles, enwrap, or trap Aß and transport it into microglia for degradation. Here, we review Aß sequestration and elimination by exosomes, and discuss how this clearance machinery might contribute to AD pathogenesis and how it might be exploited for effective AD therapy.
Keywords: Alzheimer's disease; amyloid-ß; exosome; glycosphingolipid; microglia.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

