Nicotinamide and WLDS Act Together to Prevent Neurodegeneration in Glaucoma
- PMID: 28487632
- PMCID: PMC5403885
- DOI: 10.3389/fnins.2017.00232
Nicotinamide and WLDS Act Together to Prevent Neurodegeneration in Glaucoma
Abstract
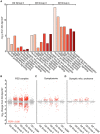
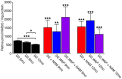
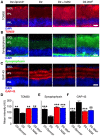
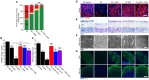
Glaucoma is a complex neurodegenerative disease characterized by progressive visual dysfunction leading to vision loss. Retinal ganglion cells are the primary affected neuronal population, with a critical insult damaging their axons in the optic nerve head. This insult is typically secondary to harmfully high levels of intraocular pressure (IOP). We have previously determined that early mitochondrial abnormalities within retinal ganglion cells lead to neuronal dysfunction, with age-related declines in NAD (NAD+ and NADH) rendering retinal ganglion cell mitochondria vulnerable to IOP-dependent stresses. The Wallerian degeneration slow allele, WldS , decreases the vulnerability of retinal ganglion cells in eyes with elevated IOP, but the exact mechanism(s) of protection from glaucoma are not determined. Here, we demonstrate that WldS increases retinal NAD levels. Coupled with nicotinamide administration (an NAD precursor), it robustly protects from glaucomatous neurodegeneration in a mouse model of glaucoma (94% of eyes having no glaucoma, more than WldS or nicotinamide alone). Importantly, nicotinamide and WldS protect somal, synaptic, and axonal compartments, prevent loss of anterograde axoplasmic transport, and protect from visual dysfunction as assessed by pattern electroretinogram. Boosting NAD production generally benefits major compartments of retinal ganglion cells, and may be of value in other complex, age-related, axonopathies where multiple neuronal compartments are ultimately affected.
Keywords: NAD+; WldS; axon degeneration; glaucoma; retinal ganglion cell.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

